Rab27a co-ordinates long-range transport of cellular cargo
New research has discovered that long-range transport in cells is co-ordinated by Rab27a.
Congratulations to Dr Wouter Kallemeijn on his recent publication to Nature Communications in collaboration with researchers at the University of Nottingham (UK), Cambridge University (UK), St. George’s University of London (UK), University College London (UK), the Spanish National Centre for Biotechnology (ES) and the University of Regensburg (DE).
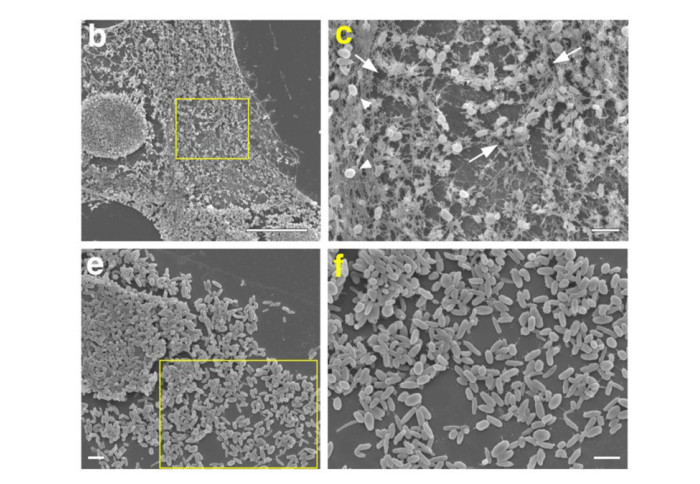
Cell biologists generally consider that microtubules and actin play complementary roles in long- and short-distance transport in animal cells. On the contrary, using melanosomes of melanocytes as a model, it was recently discovered that the motor protein myosin-Va works with dynamic actin tracks to drive long-range organelle dispersion in opposition to microtubules. This suggests that in animals, as in yeast and plants, myosin/actin can drive long-range transport.
The published work reveals that SPIRE-type actin nucleators (predominantly SPIRE1) are Rab27a effectors that co-operate with formin-1 to generate actin tracks required for myosin-Va-dependent transport in melanocytes. Thus, in addition to melanophilin/myosinVa, Rab27a can recruit SPIREs to melanosomes, thereby integrating motor and track assembly activity at the organelle membrane. Based on this, a model is suggested in which organelles and force generators (motors and track assemblers) are linked, forming an organelle-based, cell-wide network that allows their collective activity to rapidly disperse the population of organelles long-distance throughout the cytoplasm.
This work was supported by The Royal Society Newton International Fellowship grant, the European Commission Marie Sklodowska Curie Individual Fellowship grant, and Cancer Research UK.
Article supporters
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Edward Bartlett
Department of Chemistry