How to identify which proteases are cleaving a protein of interest
Congratulations to group alumni Dr Scott Lovell on his latest publication in Nature Chemical Biology
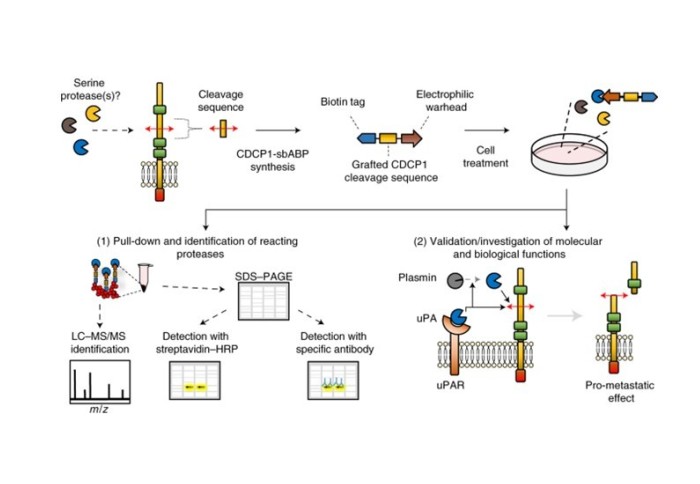
The paper ‘Substrate-biased activity-based probes identify proteases that cleave receptor CDCP1’ details work which was carried out when Dr Lovell was an ICB CDT PhD student in the Tate group and was completed in collaboration with the group of Professor John Hooper at the University of Queensland, Australia.
The research provides a template to decipher proteolytic events in diverse settings. A known protein cleavage site is grafted onto a biotinylated activity-based probe scaffold, which is then applied to a complex biological sample for specific target protease capture, isolation and identification. Using a probe based on the CDCP1 protein, Dr Lovell et al. identified urokinase (uPA) as the master regulator of CDCP1 proteolysis, which acts both by directly cleaving CDCP1 and by activating CDCP1-cleaving plasmin. Furthermore, it was demonstrated that uPA-mediated CDCP1 proteolysis promotes metastasis in preclinical in vivo models thus highlighting CDCP1 cleavage as a potential target to disrupt cancer.
During his PhD, Scott was awarded an RSC travel mobility grant to fund a short-term research project in the lab of Professor Judith Clements at the Translational Research Institute in Brisbane, Australia. During this placement Scott was introduced to co-first author Dr. Thomas Kryza and Professor Hooper. The project was initiated after an informal discussion between Dr Lovell and Dr Kryza – a real testament to the success of international placements for PhD students and to early career researchers forming interdisciplinary collaborations.
Congratulations to all involved!
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Jennie Hutton
Department of Chemistry