A Novel Subcutaneously Implanted Device for Cardiovascular Assessment
Hamlyn researchers proposed a novel implanted device for cardiovascular assessment (this work was selected as the latest AIS inside back cover story).
Implantable devices are natural substitutes for wearable monitoring platforms currently in use today. Intra-body monitoring offers a direct connection to the body at the cellular and tissue levels that has no precedent in wearable technology.
When it comes to cardiovascular assessment, monitoring of intra-body cardiovascular parameters can benefit from implantation of miniature devices close to anatomical targets. It thus overcomes the signal attenuation barriers related to the propagation towards body surface while allowing localised sensing at the target site with higher precision.
With proper electronic miniaturisation, packaging, robustness, and power consumption reduction, such implantable devices can harvest enough energy from the surrounding environment for proper operation.
A Novel Subcutaneously Implanted Device for Cardiovascular Assessment
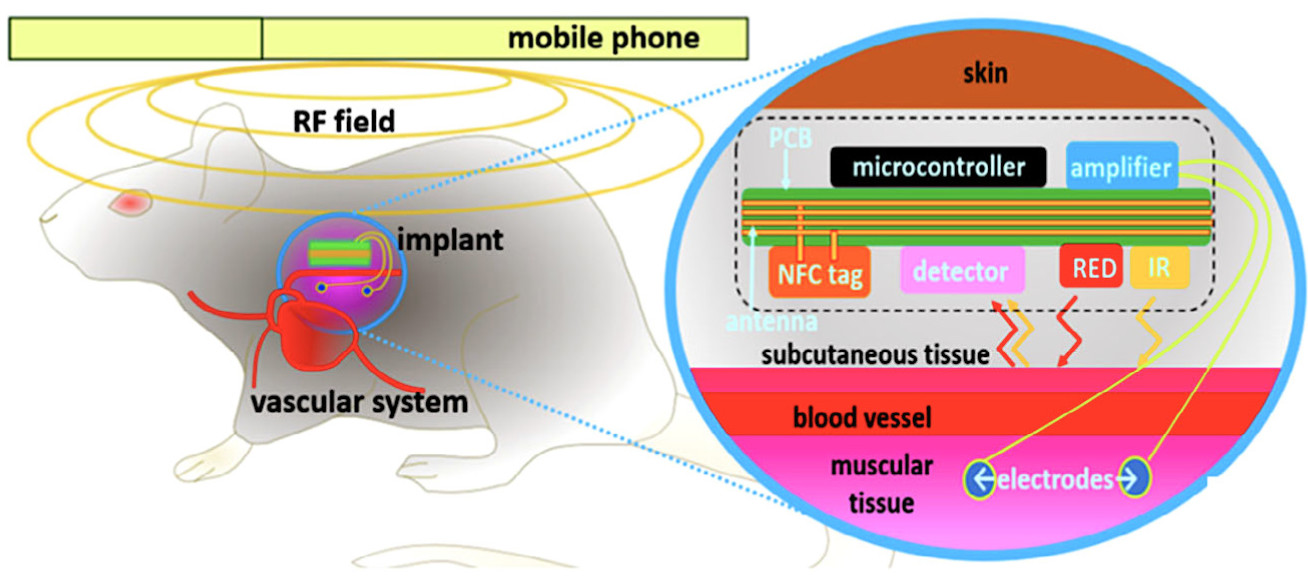
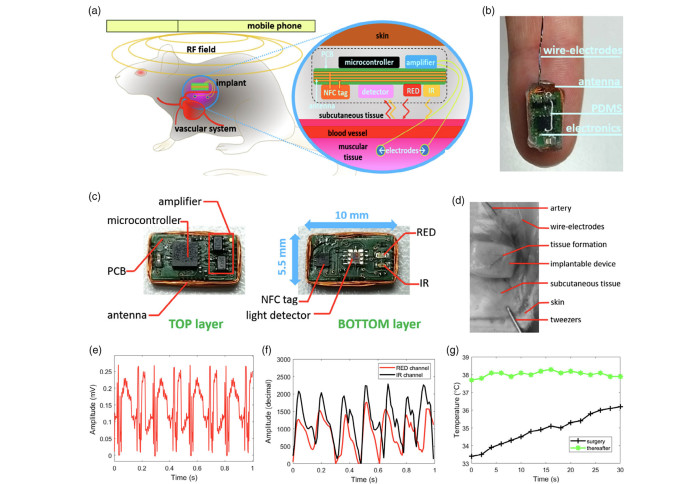
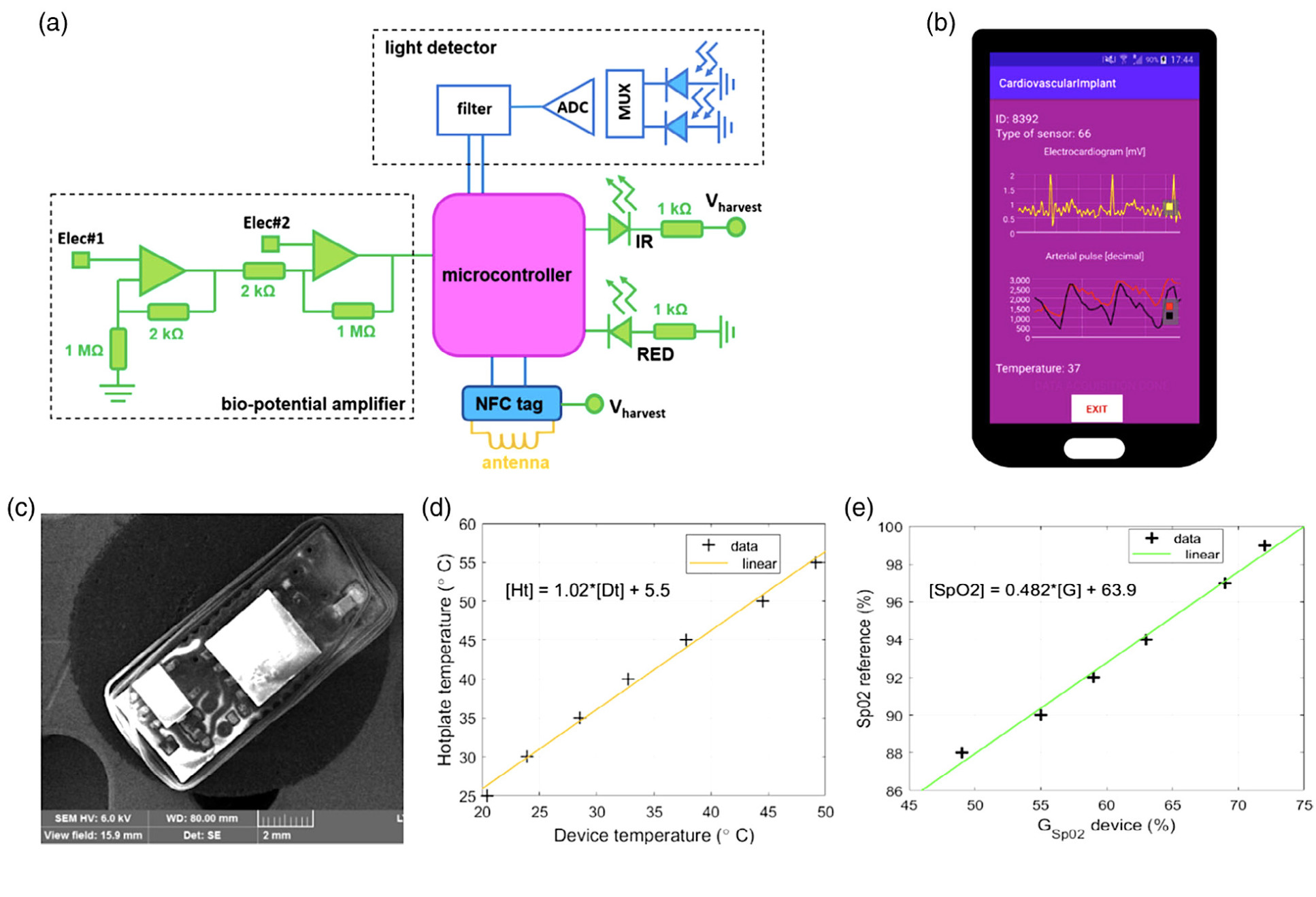
With the hope to contribute towards the development of implantable devices for cardiovascular monitoring, our researchers at the Hamlyn Centre proposed a novel near-field communication (NFC)-powered implantable device with acquisition channels for electrocardiogram, arterial pulse, and temperature measurements.
The electronic components responsible for the interface with the biological tissues are assembled on a different board layer (bottom) relative to the control and communication (on top). An additional layer of transparent elastomer covers the entire device for mechanical stability and biocompatibility with the animal tissues (See the illustration image on the cover photo).
Animal Trial Intervention and Follow-up
Implantation of the devices on the rodents took place inside a controlled trial experiment, with subcutaneous deployment of two equivalent devices on opposite sides of the dorsal region of the animals through surgery.
During surgical intervention, the animals remained anaesthetised in the prone position over the operation table, with isoflurane and oxygen supplied through a close-fitting mask. After surgical site closure, immediate NFC readings were taken externally by a phone in-close contact with the dorsal body surface of the animal.
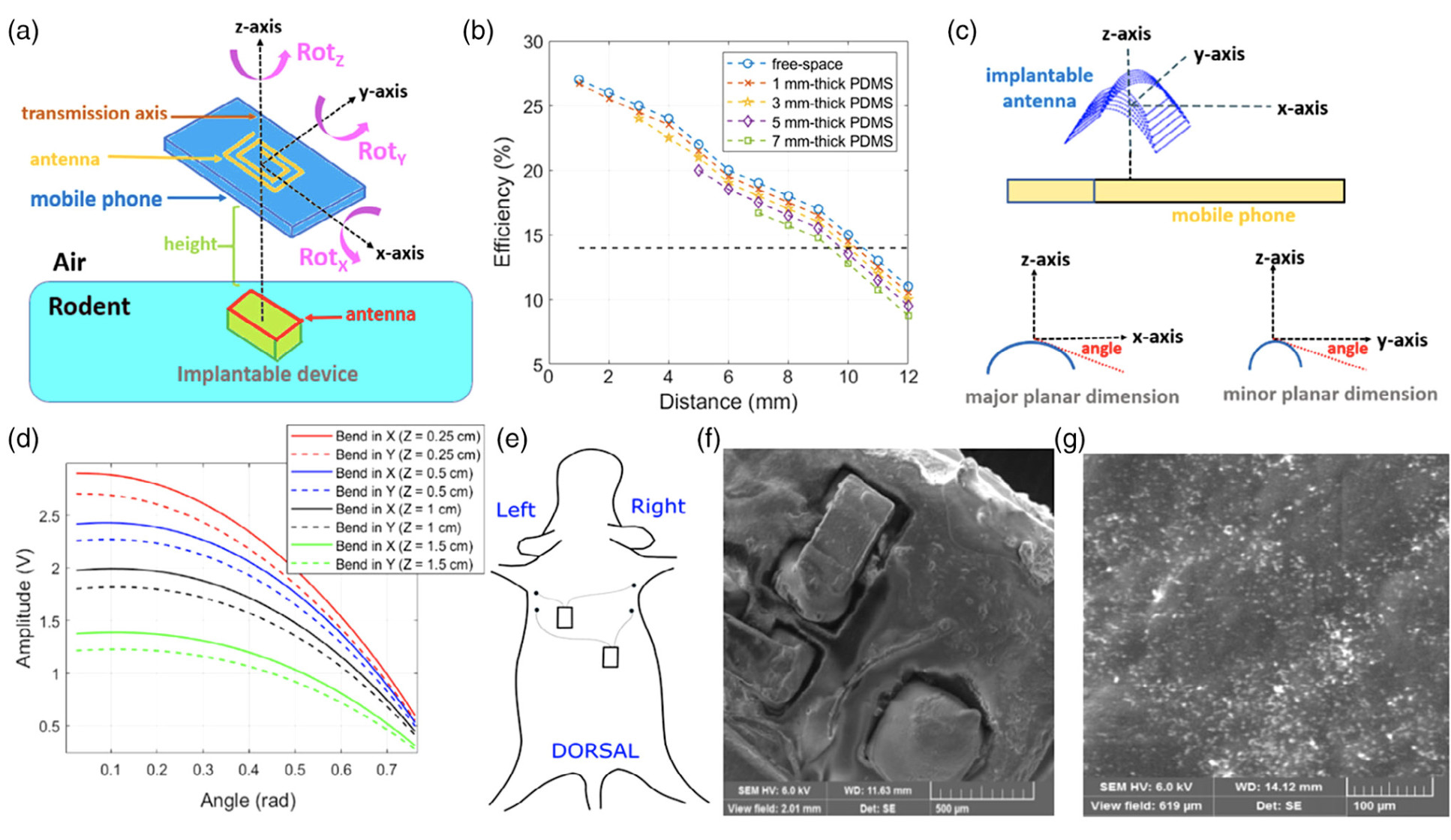

The implanted device has been successfully deployed inside rodents for a 72-hour trial period to assess external powering and data communication in living animals.
Overall, the experimental results obtained by this device demonstrated the potential for providing more reliable diagnostic information than that of external wearable devices.
This feasibility study on subcutaneously implanted devices in male rodents for cardiovascular assessment through NFC Interface has been selected as the inside back cover story of the latest Advanced Intelligent Systems Journal in June 2021.
This research was supported by EPSRC Programme Grant “Micro-robotics for Surgery (EP/P012779/1)” and EPSRC "Smart Sensing for Surgery (EP/L014149/1)" (Bruno Gil Rosa, Salzitsa Anastasova-Ivanova and Guang-Zhong Yang, "Feasibility Study on Subcutaneously Implanted Devices in Male Rodents for Cardiovascular Assessment Through Near-Field Communication Interface", Advanced Intelligent Systems, 3 (6), June 2021).
Article supporters
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Erh-Ya (Asa) Tsui
Enterprise