Rapid breast cancer screening technology could stop unnecessary chemotherapy
by Ian Mundell
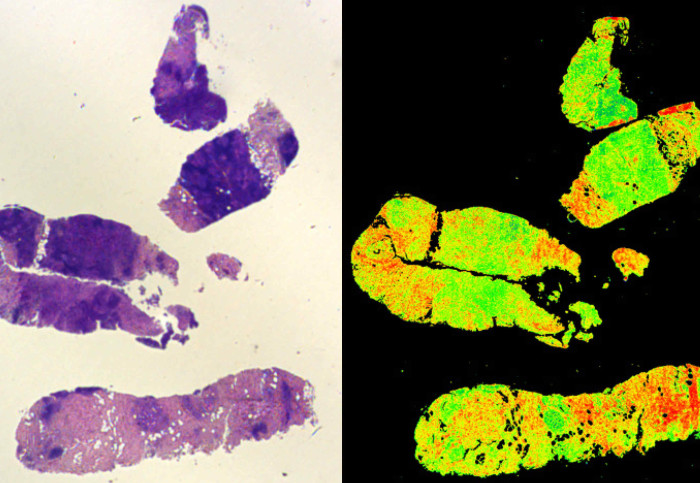
A traditional stained biopsy (left) and the Digistain method (right)
Startup company Digistain has used technology developed at Imperial to help doctors choose the best treatment for women with breast cancer.
A rapid screening system designed to prevent breast cancer patients from undergoing unnecessary chemotherapy is about to be made available in the UK, thanks to a startup working with technology developed at Imperial. The company, called Digistain, took on the patent for a biopsy imaging technique invented in the Department of Physics and used it to develop a new diagnostic tool.

“Initially we were looking at grading tumour biopsies, helping the pathologist with the initial diagnosis,” says Dr Hemmel Amrania, part of the team that developed the technology and now chief executive of Digistain. “What we learnt is that it has far more impact if we apply that technology further downstream in the patient’s care pathway. So, we are solving a slightly different problem with the same technology.”
The method, developed by Dr Amrania together with Professor Chris Phillips in the Department of Physics, analyses tumour biopsy samples by measuring unique spectroscopic signatures that highlight the chemical changes linked to the onset of cancer. When analysed with a computer program, the severity of the disease can be assessed.
After getting funding and pushing things through under our own steam, we got more commercial traction. Dr Hemmel Amrania Digistain
This approach was tested in collaboration with the Department of Surgery and Cancer, and found to be an extremely reliable indicator of breast cancer progression. It also represented an improvement on the conventional approach of staining the biopsies with vegetable dyes and assessing them by eye, since it quantified disease progression rather than leaving it to human judgement.
The technology was patented, and work began to develop a prototype for use in clinics. After options for licensing the technology to an industrial partner were explored, the College released the patent to Professor Philips and Dr Amrania, and they took the project forwards. “It was only after getting funding, and pushing things through under our own steam, that we were able to get more commercial traction,” says Dr Amrania.
What doctors want
An important part of this process was talking with oncologists about their priorities. “We asked them about the specific patient groups they struggle with, and the clinical challenges they face,” says Dr Amrania. “What we learnt is that they struggle with identifying hormone-positive, HER2 negative cancer patients who can safely forego chemotherapy.”

In these cases, hormone treatment alone will be enough to keep their cancers at bay, without the need for additional chemotherapy, a treatment that is expensive for the National Health Service and often gruelling for patients. In some cases, it’s the chemotherapy rather than the cancer that shortens lives.
“So we pivoted in terms of application of the core technology, tailoring it for a specific purpose and working out which patients to target,” Dr Amrania says. “At the research level, we recalibrated the tool for this specific purpose, but we didn’t have to change the underlying technology.”
Currently the vast majority of breast cancer biopsies are tested using expensive and time consuming diagnostics. But while these methods are accurate, they take many days or weeks to deliver a result, if not longer if samples have to be sent abroad for analysis, as is typically the case from the UK.
We've streamlined biopsy analysis so that patients are not left agonising for weeks about what their treatment will look like. Dr Hemmel Amrania Digistain
In contrast, the Digistain method typically returns a result in an hour at a fraction of the cost. This means that care decisions can be taken rapidly, and patients spared the anxiety of waiting to hear about their next treatment.
Digistain’s method has been validated in a study involving more than 800 patients, and was subsequently cleared by the UK’s Medicines and Healthcare Products Regulatory Agency. Patients in India and the USA are already using the system, and negotiations are underway for its introduction by selected National Health Service trusts.
“We are bringing this diagnosis back into the UK, with a British innovation that allows us to streamline biopsy analysis so that patients are not left agonising for weeks about what their treatment will look like,” Dr Amrania says.
Meanwhile. Digistain has been selected to receive one of Innovate UK’s highly competitive Smart awards. This money will allow the technology to be adopted across the UK, and have a wider social impact.
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Ian Mundell
Enterprise