UV-light activated probe finds new interactions in living cells
New research has discovered novel protein-protein interactions using a technique that does not require genetic manipulation of cells
Past and present Tate group researchers Drs Fedoryshchak, Gorelik, Shen, Shchepinova and Pérez-Dorado have published a study in Chemical Science.
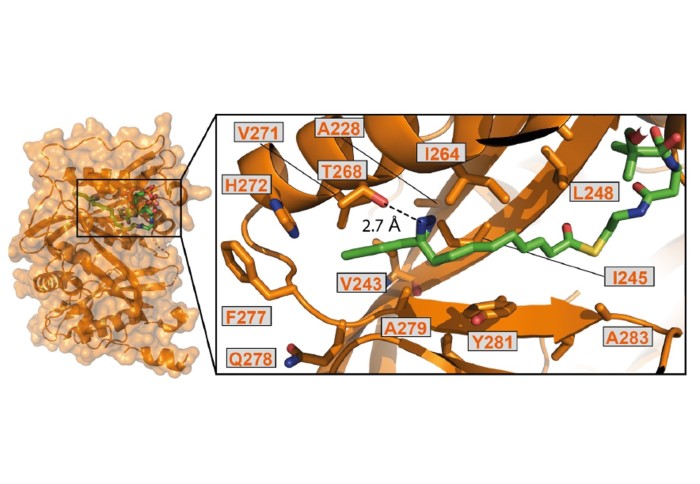
The paper describes a method to precisely install a photoactivatable group in place of a natural protein post-translational modification. UV-light activation causes the new functionality to become very reactive and crosslink two interacting proteins into a single molecule. Such crosslinks then allow researchers to accurately identify pairs of interacting proteins. The study describes several new protein-protein interactions identified in this way. The advantages of the method described is that it does not require any genetic modification while capturing protein interactions in living cells.
The study used the new technique to investigate proteins that interact with the enzymes NMT1 and NMT2. The research offers a proof of concept that these probes can be used to explore the post-translational modification interactome.
This research was funded by Cancer Research UK, the Engineering & Physical Sciences Research Council (EPSRC) and by the Biotechnology and Biological Sciences Research Council (BBSRC).
Article supporters
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Edward Bartlett
Department of Chemistry