A universal platform for chemically induced proximity-based drug discovery
The Tate group has developed a chemical–genetic platform called Site-specific Ligand Incorporation-induced Proximity (SLIP)
SLIP is designed to identify actionable (or “PROTACable”) sites on any potential effector protein in intact cells. This work was published recently in Journal of the American Chemical Society, back-to-back with related work from Ryan Mehl’s lab.
Chemical inducers of proximity (CIPs) are a powerful concept rapidly reshaping the landscape of chemical biology and drug discovery. CIPs bring two or more biomolecules into close spatial proximity to selectively modulate protein function, resulting in proximity-induced pharmacology (PIP). One of the most successful applications of this strategy is in targeted protein degradation (TPD), in which a monovalent molecular glue degrader (MGD) or bivalent conjugate such as a proteolysis targeting chimera (PROTAC) mediates a ternary complex between a degradation pathway effector protein and a protein of interest (POI), directing the latter to degradation. Blockbuster molecules like lenalidomide, a MGD used in cancer treatment, brought in over $6 billion in global sales in 2023. Meanwhile, the most advanced PROTAC Vepdegestrant is now in late-stage clinical trials, signalling a wave of new therapeutics on the horizon. Despite this momentum, the narrow set of exploitable effector proteins and ligand binding sites currently limits the scope for improved selectivity, overcoming drug resistance, and access to a broader range of disease-relevant PIP mechanisms beyond TPD.
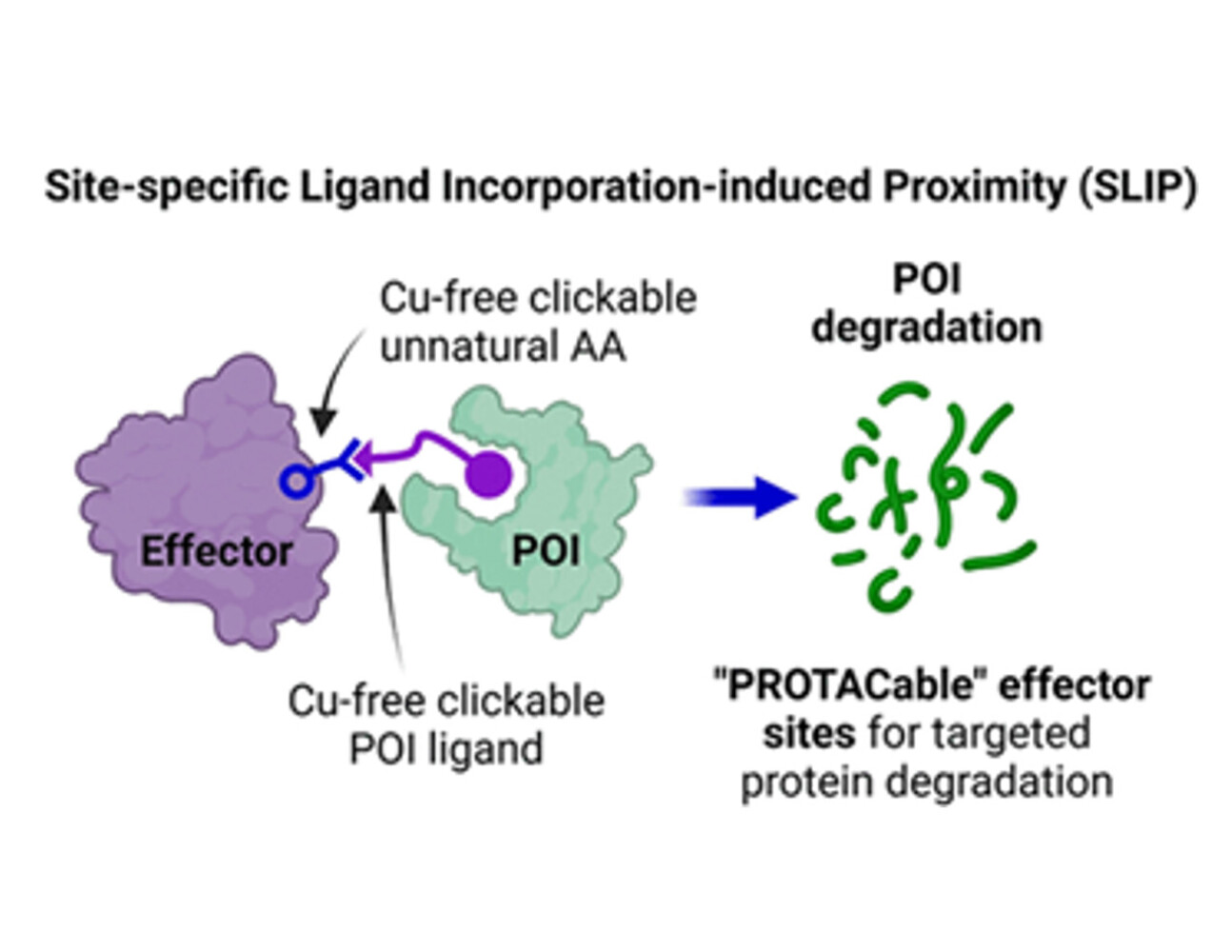
In our paper we report the first universal strategy to precisely and site-specifically induce protein-protein proximity to identify PROTACable sites on any potential effector protein in live cells, an approach we call “Site-specific Ligand Incorporation-induced Proximity”, or SLIP. SLIP uses genetic code expansion to encode an unnatural amino acid for copper-free “click” ligand ligation at a specific effector site in intact cells, enabling in situ formation of a covalent PROTAC-effector conjugate against a target protein of interest. Modification at actionable effector sites drives degradation of the targeted protein, establishing the potential of these sites for TPD. SLIP enabled identification of multiple novel potentially PROTACable E3 and E2 sites which are competent for TPD. SLIP adds a powerful approach to the proximity-induced pharmacology (PIP) toolbox, enabling future effector ligand discovery to fully enable TPD, as well as other emerging PIP modalities such as targeted protein stabilisation.
This work was undertaken in collaboration between the Tate lab at Imperial and the Kozakov lab at Stony Brook University, US. It was supported by a Marie Skłodowska-Curie Action, the Health@InnoHK Program, CSC, ORF (HKU), NIH grants, Cancer Research UK and EPSRC.
Congratulations to Zhangping and the team!
Main image by Dr Jana Volaric.
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Edward Bartlett
Department of Chemistry