Affinity-based protein profiling of MDM2 inhibitor Navtemadlin
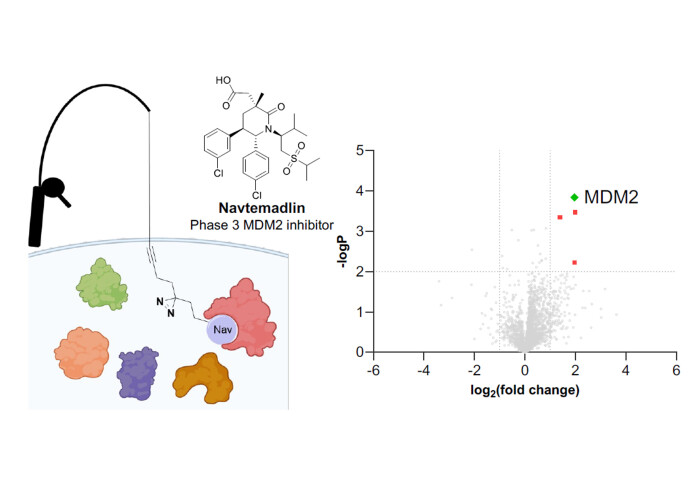
We report the synthesis and validation of probes of MDM2 inhibitor Navtemadlin
New research published in Chemical Science in collaboration with Professor Andy Wilson's groups at the Universities of Leeds and Birmingham and the Barnard and Tate groups at Imperial focuses on an anti-cancer drug.
Navtemadlin is a potent inhibitor of the p53-MDM2 protein–protein interaction, which plays a critical role in the proliferation of p53-wildtype tumours. Whilst Navtemadlin has progressed to multiple Phase III clinical trials in oncology, little has been disclosed regarding its target selectivity in cells. This work involved the synthesis and validation of photoactivatable clickable probes of Navtemadlin, and their application to de novo target discovery for Navtemadlin through affinity-based protein profiling. MDM2 was robustly identified as the main target, across two cell lines, with a high degree of selectivity for the target protein. Additionally, whole proteome profiling experiments were conducted across different time points to confirm p53-mediated phenotypic activity and uncover novel expression patterns for key proteins in the p53 pathway.
Congratulations to Dr Amrita Date and all involved in this research!
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Edward Bartlett
Department of Chemistry