New small molecule locks out antibiotic efflux in MRSA
New preprint from our lab, in collaboration with Andy Edwards and Nate Traaseth: a novel small molecule which locks out antibiotic efflux in MRSA.
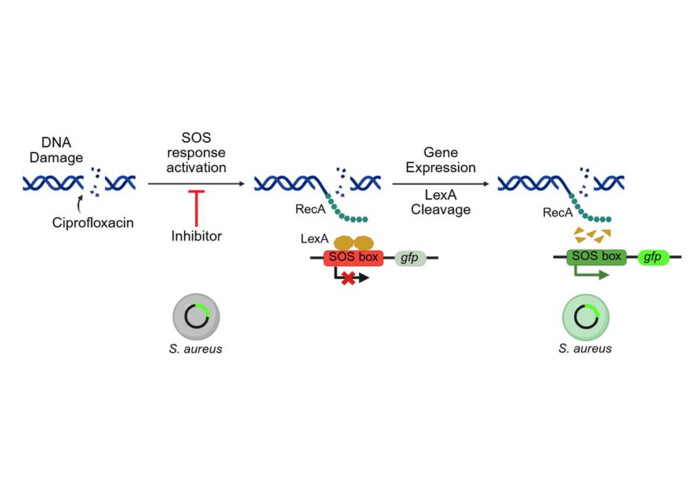
Antibiotic resistance remains one of the greatest threats to global health. Efflux pumps like NorA in Staphylococcus aureus eject antibiotics from bacterial cells, protecting the pathogen and fuelling resistance.
In a high-throughput screen for inhibitors of the ciprofloxacin-induced SOS DNA repair pathway in MRSA, we uncovered an unexpected hit series. Medicinal chemistry optimisation and target ID led to IMP-2380, the most potent NorA inhibitor reported to date.
Using cryo-EM at 2.5 Å, we captured the mechanism in atomic detail: IMP-2380 captures NorA in an outward-open conformation, making NorA invisible to ciprofloxacin and locking the door on antibiotic efflux.
The result?
✨ Nanomolar potentiation of ciprofloxacin in vitro
✨ Suppression of resistance by blocking the SOS response
✨ A 100-fold reduction in bacterial burden in a murine MRSA infection model
IMP-2380 is the first in vivo-active, structurally defined NorA inhibitor, showing the potential to drug efflux with small molecules to restore antibiotic efficacy and slow the evolution of resistance. With many thanks to all the scientists who contributed to this work at Imperial College London, New York University and the Drug Discovery Unit, University of Dundee, including 1st authors Janine Gray and Lizzie Ledger, and Tiffany Suwatthee.
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Edward Bartlett
Department of Chemistry