Chemistry to me. By Joshua Davies (Cohort 1 - Intake 2019)
“I hope he gets a job in Boots”. My auntie Beryl says while excitedly telling my cousins about my career path.
“Different type of chemist auntie” I respond with a laugh. After which I attempt to explain what a research chemist actually is.
Describing your career can often be challenging as a chemist, especially when you don’t know the scientific background of the person you’re talking to. We find ourselves using buzz words like “drug design”, “pharmaceuticals” and “cancer treatments” in an attempt to romanticise the science. Hoping the listener will understand.
There’s nothing wrong with a bit of romance. I think science is inherently beautiful. It deserves all the poetic metaphors you can throw. In the past I have compared the synthesis of a chemical to architecture, with the venture of forming an interesting molecule being synonymous with designing and building a magnificent building. But as lovely as these metaphors may be, what do we actually do on a day-to-day basis?
I’m Josh. I’m a third year PhD student as part of the CDT for Next Generation Synthesis in Imperial College London. My research is looking at new methods for the formation of amide bonds.
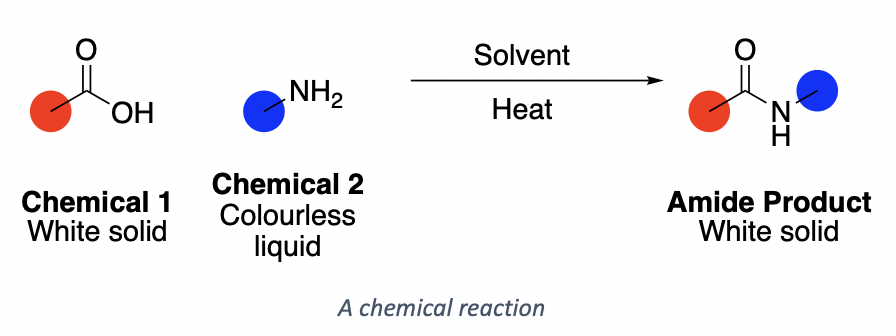
That’s the formal description. Doesn’t reveal much to the non-chemist. So what does this involve? Well, mixing chemicals to see what happens. Day by day, I dissolve a solid (usually a white powder), add a colourless liquid, stir, carry-out a purification, and form a new white powder which I hope to be different from the first.
This may seem mundane. But to the chemist, equipped with a scientific imagination, something extraordinary is happening within that reaction vial. Chemical transformations: activation, rearrangements, and bond formations, often invisible to the naked eye, are all ongoing within this colourless solution.
“How do you know which chemicals to add?”. Well, like the architect, we know which bits fit together. Each experiment we carry out is designed with the purpose of learning something. Using the language of organic chemistry, we know what chemicals we put in, and can predict the structure of the chemicals we get out. With this knowledge, chemists (academic and industrial alike), are making ever more complicated (and useful) chemicals, and designing ever more useful reactions.
 “But how do you know the new white powder is different?”. A wonder of being a chemist today is that we have a range of amazing techniques at our disposal to give insight into what’s going on. Various properties of chemicals can be exploited to provide useful data on their structure. Nuclear Magnetic resonance (NMR) spectroscopy, infrared (IR) spectroscopy, mass spectrometry, X-ray crystallography and melting point, are just some the many methods by which we can probe the structure (and purity) of a chemical. These techniques bring substance to the chemist’s imagination, and shed light on the unknown.
“But how do you know the new white powder is different?”. A wonder of being a chemist today is that we have a range of amazing techniques at our disposal to give insight into what’s going on. Various properties of chemicals can be exploited to provide useful data on their structure. Nuclear Magnetic resonance (NMR) spectroscopy, infrared (IR) spectroscopy, mass spectrometry, X-ray crystallography and melting point, are just some the many methods by which we can probe the structure (and purity) of a chemical. These techniques bring substance to the chemist’s imagination, and shed light on the unknown.
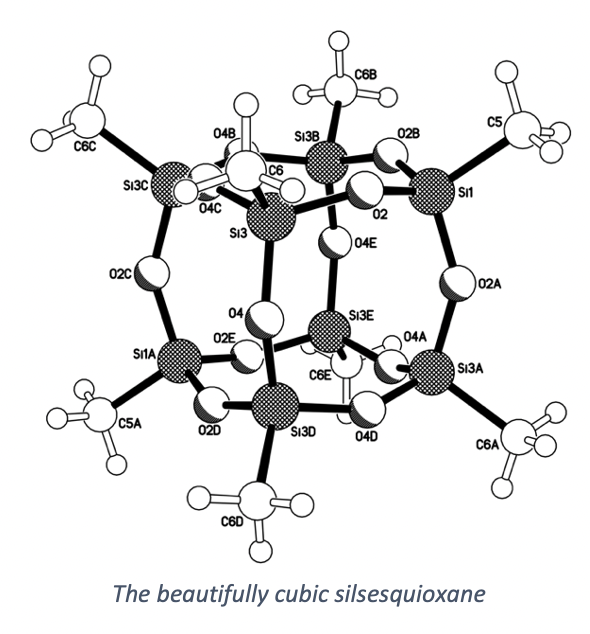
Recently, on return to the lab I unexpectedly found the most gorgeous cubic crystals (white solid) sitting at the bottom of a reaction flask. If these crystals were displayed next to table salt, you wouldn’t notice much difference. But X-ray crystallography revealed this solid to be a silsesquioxane. The cube crystals were made up of a smaller molecular cube. Beautiful.
It’s the moments like these that makes synthetic chemistry so wonderful. Taking the simple white solid and using modern techniques to reveal the most amazing of structures.
And when we are faced with such excitement, it’s almost difficult not to use metaphors.
Joshua Davies is a third year PhD student at the CDT React and is currently undertaking a research project titled Automated Direct Amidation of Carboxylic Acids using Silicon-Based Reagents with Prof Chris Braddock.
