The burden of life-long disabilities arising from brain injury during newborn period is far higher in low- and middle-income countries than in high-income countries. The vast majority results from perinatal asphyxia or infection, and often a combination of these.
Whilst cooling therapy reduces death and disability after neonatal encephalopathy in high-income countries, the safety and efficacy of cooling therapy in low and middle-income countries in unknown. Over the past decade, our work has been focused on understanding brain injury after neonatal encephalopathy in low- and middle-income countries and evaluating the safety and efficacy of cooling therapy in these settings. We have set up a consortium of large perinatal centres in South Asia with facilities for three Tesla MR imaging and spectroscopy for conducting this work.
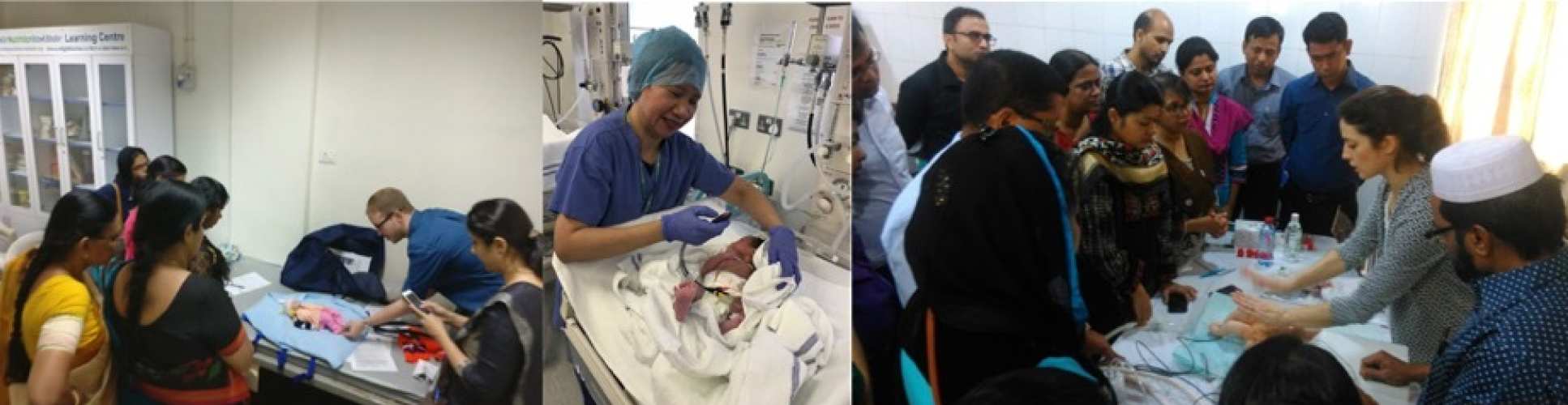
Ongoing studies
Erythropoietin Monotherapy in Neonatal Encephalopathy in Low and Middle-Income Countries (EMBRACE) - Funding: Thrasher Foundation
EMBRACE trial will recruit from several large secondary and tertiary neonatal units in India,
Sri Lanka, and Bangladesh, that cater to a low-income population. All participating neonatal
units will have facilities for providing good supportive neonatal intensive care, including
facilities for ventilation and inotropic support. Dedicated neonatal research nurses will be
appointed at each participating site. All recruiting sites will also have adequate facilities to
ensure research governance. These include a dedicated research office, video conferencing
facilities, protocols and systems for MR scanning and transport, harmonised MR scanners
with the spectroscopy sequences, trained and certified clinical staff on NICHD neurological
examination based on the modified Sarnat stage, and a neurodevelopmental paediatrician to
undertake the Bayley assessment certified for concordance with a gold standard examiner.
The follow up duration is 18-22 months and the planned trial period is 48 months.

Prevention of Epilepsy by reducing Neonatal Encephalopathy (PREVENT) study - Funding: NIHR
Please see the PREVENT study website for more details
Summary of the PREVENT study (watch the video link below: Courtesy Sandhya Ramesh, PRINT, Bangalore)
Completed studies
Hypothermia for Encephalopathy in Low and Middle Income Countries (HELIX) Trial (Funding: NIHR and Weston Garfield Foundation) trial
Although cooling is the standard of care for neonatal encephalopathy in high-income countries, the safety and efficacy of cooling in low- and middle-income countries (LMICs) - which shoulder 99% of the disease burden - is unclear. A total of 408 encephalopathic babies will be randomised to cooling or usual care, from three countries (India, Sri Lanka and Bangladesh). Outcome measures are based on three Tesla MRI and MR spectroscopy at two weeks after birth, and death/neurodisability at 18 months after birth. HELIX is the largest ever cooling trial in the world, and the only such trial to use MR spectroscopy biomarkers across multi-vendor platforms.
See the HELIX trial video on the last trial baby (No:408) (Recruited in Feb 2019)
HELIX Trial
Bayley assessment of the final (408) baby at Bangalore at the roof top in a remote village in Karnataka (Sept 2020)
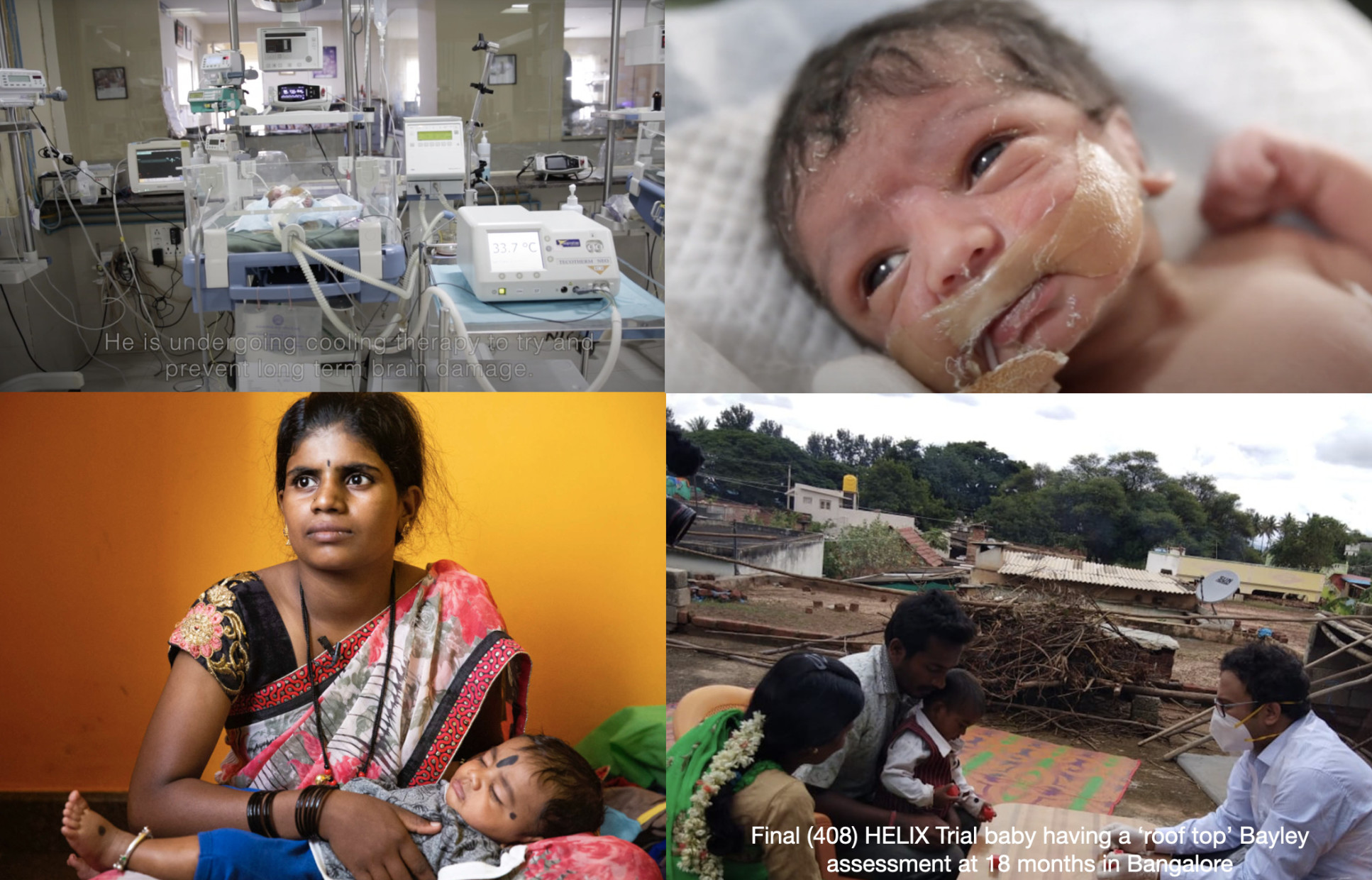
HELIX Trial Part 2
Contact us
Centre for Perinatal Neuroscience
Department of Brain Sciences
5th Floor, Hammersmith House
Hammersmith Hospital
Du Cane Road
London, W12 0HS
Ms Martin, Caroline J
Clinical Trials Manager, Centre for Perinatal Neuroscience
Room 515, Hammersmith House
+44 (0) 78 1753 2977
c.martin1@imperial.ac.uk


