Pharmaceutical manufacturing waste is an important and neglected source of antimicrobial contamination in the environment that can result in the expansion of antimicrobial-resistant microbes. Countries such as India, one of the world's main producers of Active Pharmaceutical Ingredients (APIs) are at particular risk.
Studies indicate that pharmaceutical effluent waste in specific areas of India is associated with excessively high concentrations of antimicrobials in the surrounding environment and the increased frequency of antimicrobial resistance (AMR) in environmental water. The impact of such waste in other areas is unknown. There are currently no international standards to limit antimicrobials in antimicrobial manufacturing waste (AMW).
Such pollution from manufacturing creates environments that are spawning grounds for antimicrobial resistance in bacteria. New factors for genetic resistance can evolve through mutation, and, together with already-existing resistance traits be enriched by selection. Due to the ability of bacteria to transfer genetic material on mobile genetic elements, AMR genetic traits may be transferred from environmental bacteria to harmful bacteria (pathogens). This can occur within the environment or within animals and humans but will have potentially harmful health consequences for everyone, regardless of where the pollution occurred.
The impact of pharmaceutical manufacturing pollution on AMR
HCWH Europe video on the environmental impact of pharmaceutical manufacturing pollution
Focusing on Chennai and Puducherry
Focusing on Chennai and Puducherry, we will investigate the link between antibiotics manufactured by different methods, contamination levels in manufacturing wastes and downstream locations. We will sample for antibiotics and antimicrobial resistance in waters, sediments, animals, and humans, contrasting our findings with control upstream environments.
These two regions in India have a significant number of pharmaceutical companies (both active pharmaceutical ingredient manufacturers and formulation companies) producing a range of products including amoxicillin, cephalosporins, macrolides, tetracyclines, and fluoroquinolones.
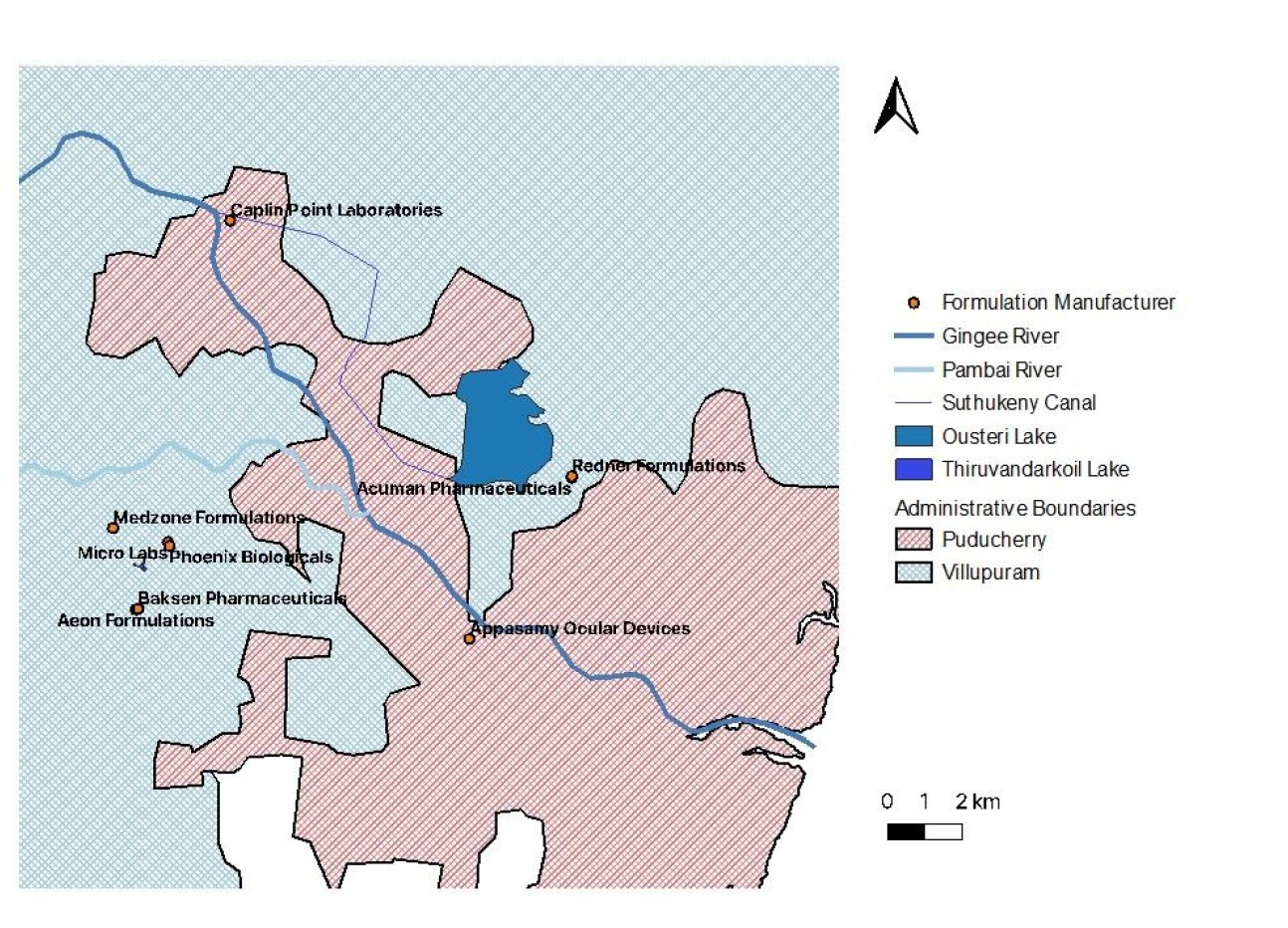
Map of antibiotic producers in Puducherry
We will validate techniques to detect and measure different antibiotics in environmental samples and undertake state of the art bacterial, genomic and metagenomic studies on the same samples to identify AMR bacteria and the genetic elements conferring resistance. This will provide a library of AMR risk in this region of India. AMR data will be integrated with locations of sampling and levels of antibiotic contamination to determine the degree of association between these factors.
Armed with this information, our work will provide a platform to advise on the risk posed by antimicrobial manufacturing waste and ultimately identify the need for risk-reducing investments by industry to deal with the proliferation of AMR. The project will develop novel analytical methods for the quantification of APIs related to antibiotic manufacturing and, novel approaches to quantify and characterise the penetration of AMR. We will deliver mass balance methods and risk assessment tools that predict concentrations and risk, based on production loads of antimicrobials, and develop policy recommendations for international environmental standards for antimicrobials in manufacturing effluents and receiving environments.
Global implications
The global implications of this work relate to the larger pharmaceutical industry that purchase antibiotic ingredients, as well as medical professionals and patients. There may be cause to consider that the low cost of antibiotics should be balanced by a need to maintain the environmental performance and sustainability of their manufacturing methods. Given the significant industrial emissions of antibiotics repeatedly documented in India, our work will encourage and support the Indian government in its determination to monitor and regulate antibiotic discharges from manufacturing, whilst supporting global efforts for tackling AMR.
For further information regarding our work packages, please visit our Research Page
Contact us
For further information on the project, please contact:
Prinicpal Investigator: Professor Nick Voulvoulis
Tel: +44 (0)20 7594 7459
Centre for Environmental Policy: Weeks Building, 16-18 Prince's Gardens, London, SW7 1NE