Multiphoton fluorescence microscopy for clinical applications
EPSRC Healthcare Technology Challenges for Engineering (EP/K020102/1)
At present, the diagnosis of many types of disease must be confirmed by histopathology before treatment can begin. This entails the physical removal of a small tissue specimen from the patient that is then processed, sliced, stained and analysed by an expert using an optical microscope. The collection of tissue is subject to sampling error, i.e. the diseased tissue may be missed, and the time required for processing and analysis can delay treatment. It would desirable for physicians to be able to make an immediate diagnosis during examination of the patient.
When biological tissue is illuminated with light at appropriate wavelengths, certain naturally occurring biomolecules can absorb this excitation energy and emit autofluorescence that can be used to provide high resolution images of biological tissue. Being label-free, autofluorescence avoids potential hazards associated with externally applied contrast agents. The properties of this light can provide information about the molecules that emitted it and this enables imaging of the biochemical or structural changes in tissue associated with disease, which can aid diagnosis.
A key challenge for optical imaging in tissue, however, is optical scattering, which limits the depth at which optical images can be obtained. Multiphoton microscopy entails illuminating tissue with light at twice the wavelength (half the photon energy) that is usually absorbed. If the incident intensity is high enough, then the tissue can absorb two photons simultaneously to produce each autofluorescence photon. This permits excitation of tissue at longer wavelengths (which undergo less scattering and permit deeper imaging) and also provides 3-D imaging. This is because fluorescence is only efficiently produced in the focal plane where the intensity is highest. During this research project we developed two novel multiphoton imaging instruments for clinical applications.
Hand-held multiphoton microscope with built-in axial motion compensation
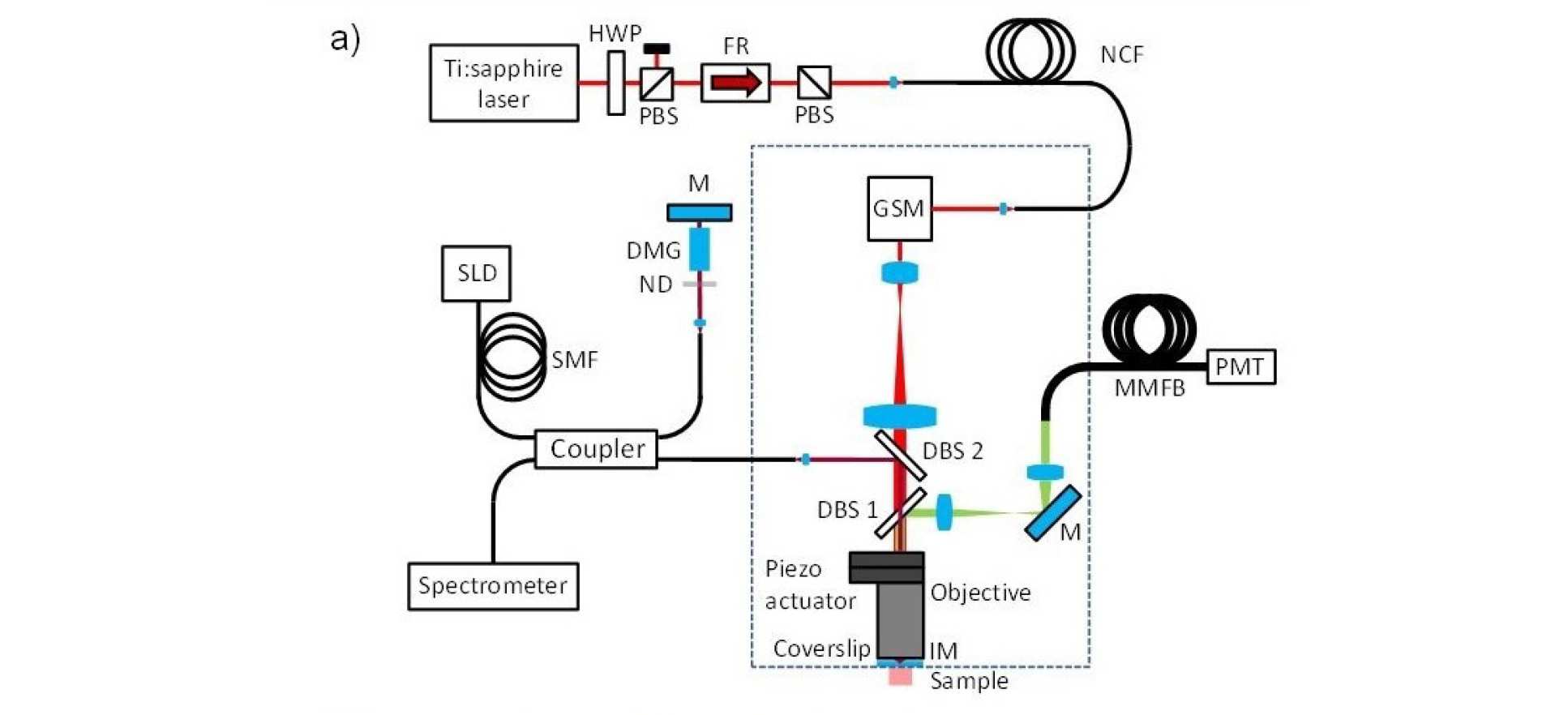
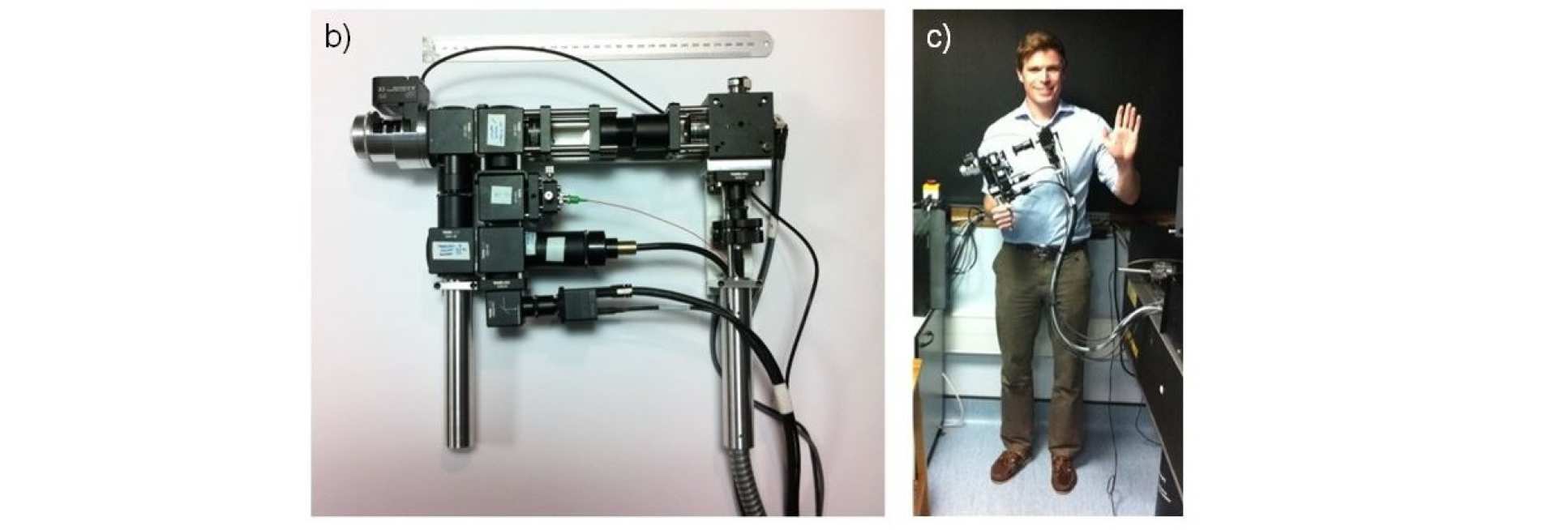
When trying to perform sub-cellular imaging in vivo, even relatively small motions of the multiphoton scanner or sample can cause large motion artefacts in the final image. In this instrument, we use optical coherence tomography to track the surface of the skin, see figure 1. This position information is then fed back in real time to control the position of the microscope objective in order to achieve axial motion compensation [1]. The performance of this hand-held multiphoton microscope was tested on an ex vivo skin sample, see figure 2, and successfully applied to imaging human chest skin in vivo with the volunteer sitting and the instrument hand-held.
Working with the Centre for Photonics and Photonic Materials at the University of Bath, we tested the use of a new class of optical fibre - called negative curvature fibre - and demonstrated that it can be used to deliver ultrafast laser radiation for multiphoton microscopy without nonlinear spectral or temporal broadening of the excitation pulses [2].
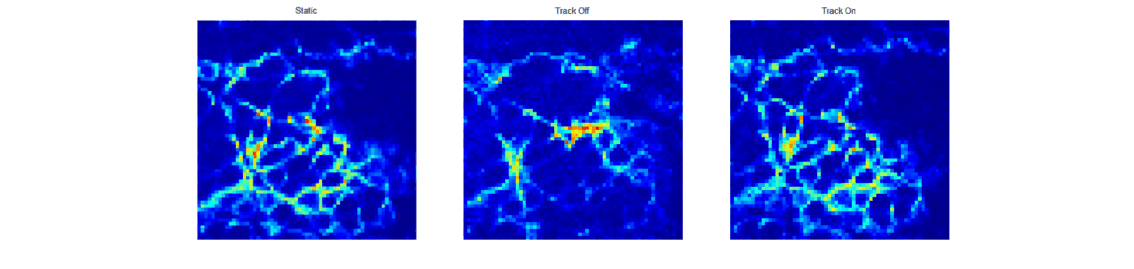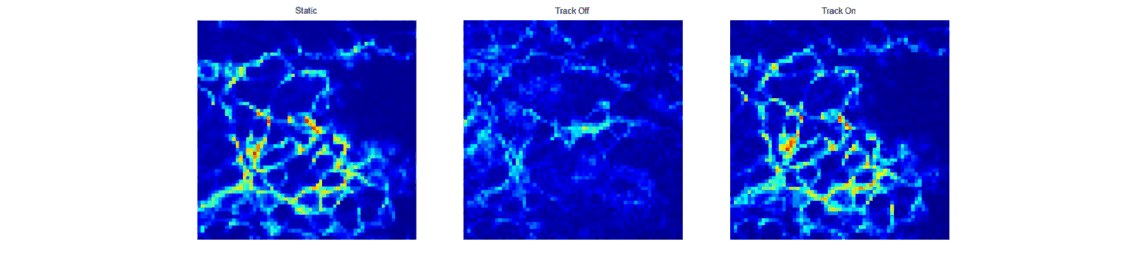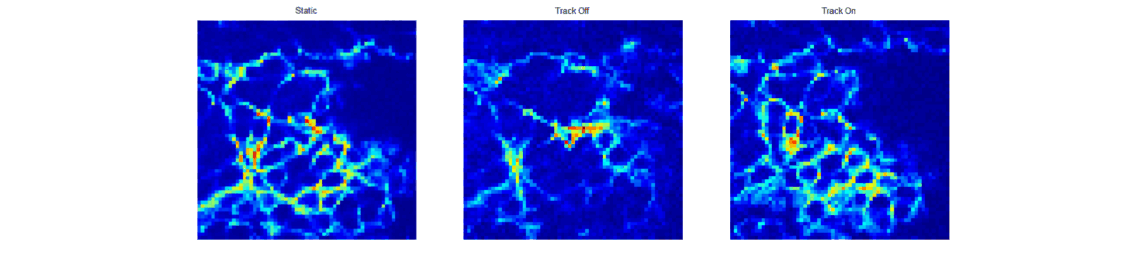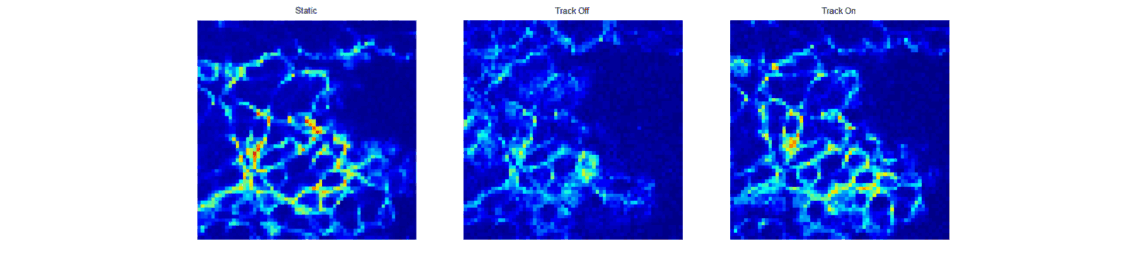
Adaptive multiphoton endoscopy
In order to provide high resolution sub-cellular imaging within the body, it is desirable to construct the smallest possible multiphoton endoscope. Building on previous work in our group (A. Thompson et al, doi:10.1364/OL.36.001707), we use an interferometer to measure the optical path length of every core within a bundle of single mode fibres and then use a spatial light modulator (SLPM1) to correct for the optical path length variations across the bundle, see figure 4. By then dynamically adding tip, tilt and focus to the phase-front generated at the distal tip of the bundle, it is possible to generate a scanning excitation spot at the distal tip of the fibre without the use of any distal optical components. This approach offers the potential to build extremely small multiphoton endoscopes without the need for miniaturised distal optical elements.
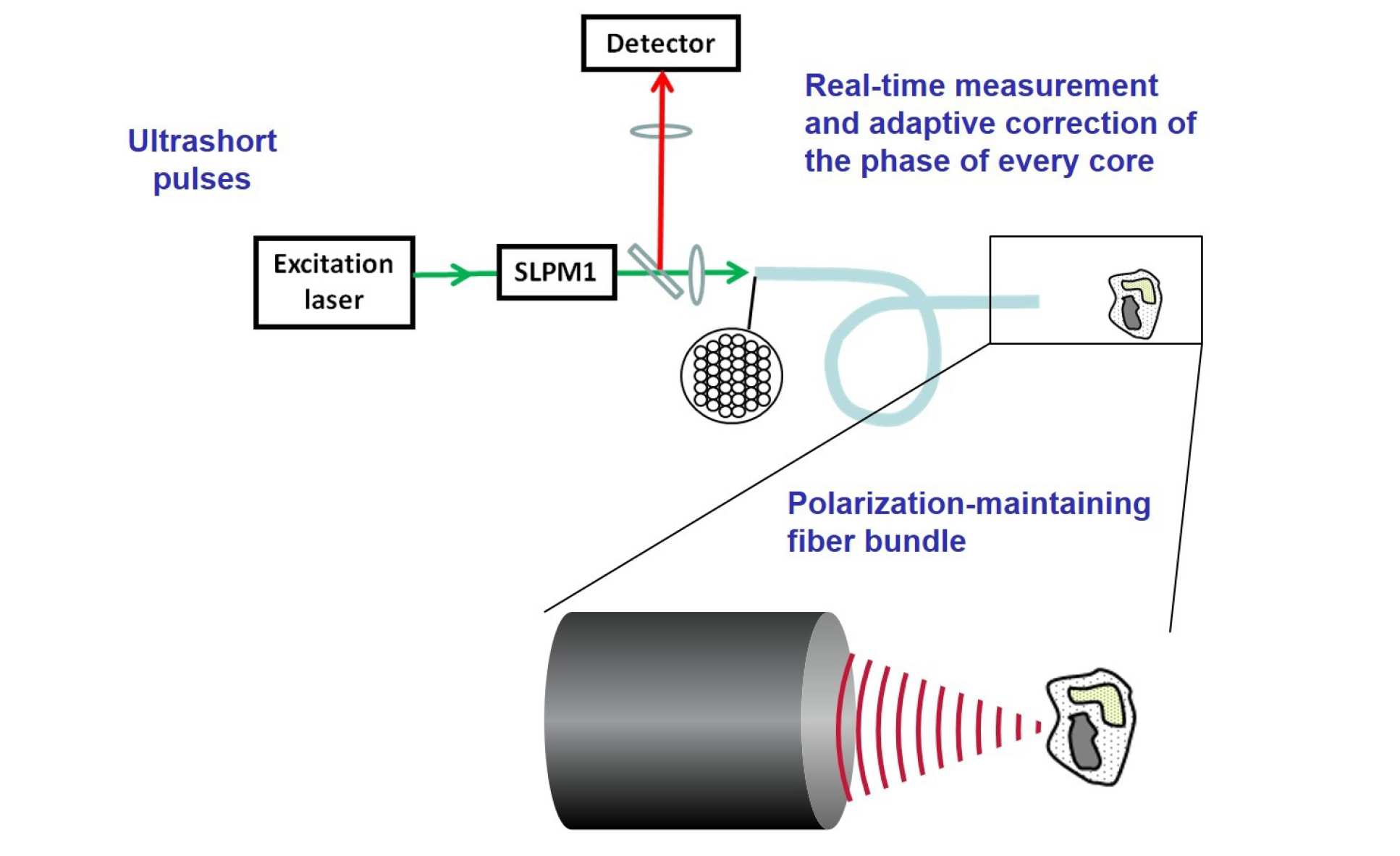
Figure 4. Schematic of method for generating a focused beam at the distal tip of a bundle of single mode fibres.
During this project, the Centre for Photonics and Photonic Materials at the University of Bath developed a polarisation maintaining fibre bundle with 96 single-mode cores [3] and we used this bundle to demonstrate the first multiphoton adaptive endoscopy using a polarisation maintaining fibre bundle [4], see figure 5. We have also demonstrated the first imaging through this fibre while the fibre is being flexed and the results are currently being written up for submission to a peer reviewed journal. This research project also led to a method to minimise group index variations in non-polarisation maintaining multicore endoscope fibres [5].
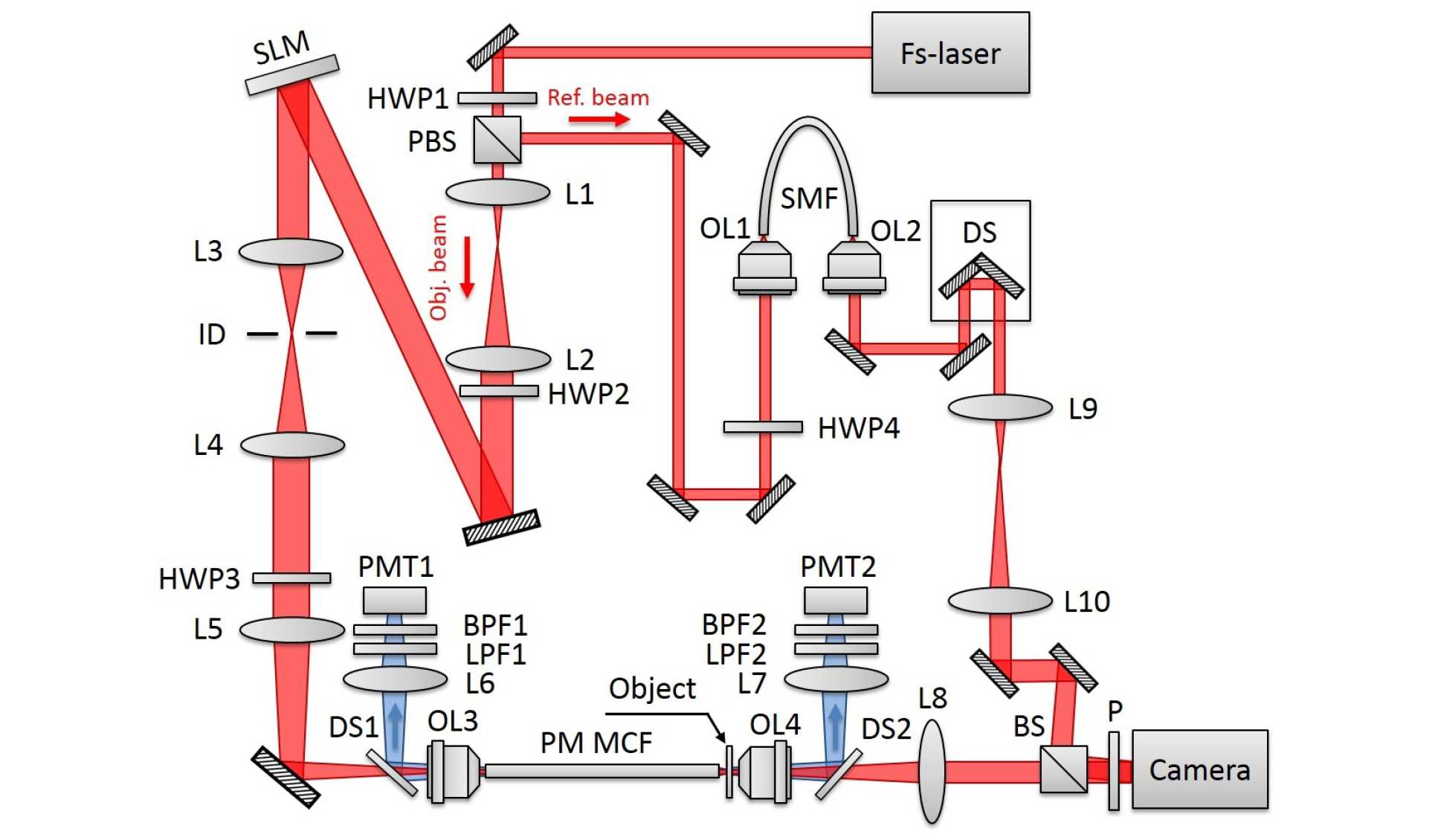
Figure 5. Experimental setup for the adaptive multiphoton endoscope.
Publications
- Fibre-coupled handheld multiphoton microscope with active motion compensation
B. Sherlock, S. Warren, J. Stone, M. Neil, C. Paterson, J. Knight, P. French and C. Dunsby
Biomedical Optics Express 6(5) 1876-1884 (2015)
http://dx.doi.org/10.1364/BOE.6.001876 - Tunable fibre-coupled multiphoton microscopy with a negative curvature fibre
B. Sherlock, F. Yu, J. Stone, S. Warren, C. Paterson, M. A. A. Neil, P. M. W. French*, J. Knight* and C. Dunsby
Accepted for publication in Journal of Biophotonics - Highly birefringent 98-core fiber
J. M. Stone, F. Yu, and J. C. Knight
Optics Letters 39(15) 4568- 4570 (2014)
http://dx.doi.org/10.1364/OL.39.004568 - Adaptive multiphoton endoscopy with a polarisation maintaining fibre bundle
Y. Kim, S. C. Warren, J. M. Stone, J. C. Knight, M. A. A. Neil, C. Paterson, C. Dunsby and P. M. W. French
IEEE JSTQE 22(3) 6800708 (2015)
http://dx.doi.org/10.1109/JSTQE.2015.2488283 - Minimizing Group Index Variations in a Multicore Endoscope Fiber
J. C. Roper, S. Yerolatsitis, T. A. Birks, B. J. Mangan, C. Dunsby, P. M. W. French and J. C. Knight
IEEE Photonics Technology Letters 27(22) 2359-2362 (2015)
http://dx.doi.org/10.1109/LPT.2015.2464377 - Adaptive multiphoton endomicroscopy through a dynamically deformed multicore optical fiber using proximal detection
S. C. Warren, Y. Kim, J. M. Stone, C. Mitchell, J. C. Knight, M. A. A. Neil, C. Paterson, P. M. W. French and C. Dunsby
Optics Express 24 (19), pp. 21474-21484, (2016)
http://dx.doi.org/10.1364/OE.24.021474 - In vivo multiphoton microscopy using a handheld scanner with lateral and axial motion compensation
B. Sherlock, S. C. Warren, Y. Alexandrov, F. Yu, J. Stone, J. Knight, M. A. A. Neil, C. Paterson, P. M. W. French, C. Dunsby
Journal of Biophotonics 11 (2), e201700131 (2018)
http://dx.doi.org/10.1002/jbio.201700131


