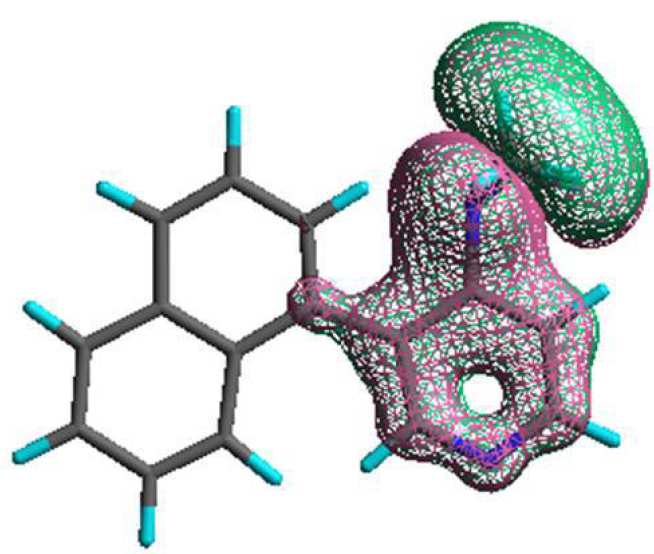
DMAP-based chiral nucleophilic organocatalysts
N,N-Dimethylamino pyridine (DMAP) and its N-oxide are widely used synthetically as nucleophilic catalysts in acylation, sulfonylation and phosphorylation reactions of amines and alcohols. We have a program to prepare and evaluate chiral DMAP and DMAP-N-oxide derivatives to achieve acylative/sulfonylative kinetic resolution and asymmetric desymmetrisation of various synthetically useful classes of alcohols and amines. The type of chiral DMAP derivatives that currently interest us are chiral by virtue of hindered rotation about an aryl-aryl bond at the 3-position of the pyridine ring (i.e. they are atropisomeric). The below image shows a molecular model of one of these catalysts.
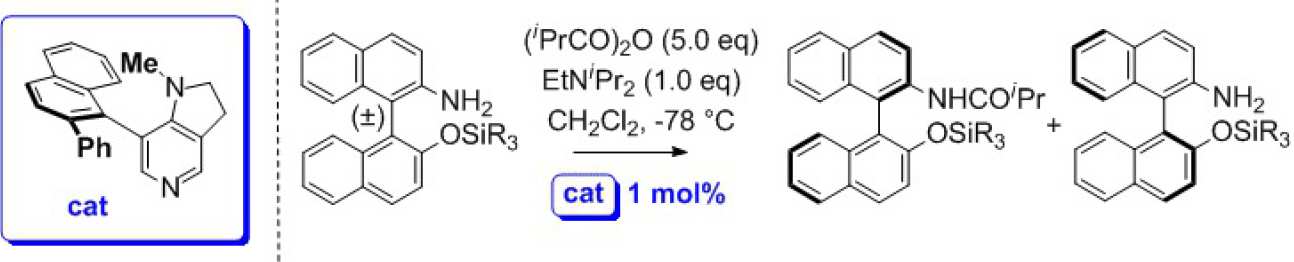
We have previously shown that these compounds are very effective for acylative kinetic resolution of various secondary alcohols using various achiral anhydrides at low temperature (see selected references below). We are currently preparing and screening a range of new structures inspired by both DMAP and DMAP-N-oxide to provide improved catalysis and also exploring their ability to mediate acylative and sulfonylative asymmetric desymmetrisation of meso-substrates, and kinetic resolution of amines. e.g.
Funded by
Collaboration with
-
Prof. Hendrick Zipse for molecular modelling of reaction transition states (Ludwig-Maximilians-Universität München, Department Chemie und Biochemie, München, Germany). Prof. Michael Whitehead for molecular modelling of dynamic rotation processes (McGill University, Department of Chemistry, Montreal, Canada).
Selected Publications
- E. Larionov, M. Mahesh, A.C. Spivey, Y. Wei, H. Zipse, 'Theoretical Prediction of Selectivity in Kinetic Resolution of Secondary Alcohols Catalyzed by Chiral DMAP Derivatives', J. Am. Chem. Soc. 2012, 134, 9390–9399 [PDF, 1.91MB];
- S. Arseniyadis, M. Mahesh, P. McDaid, T. Hampel, S.G. Davey, A.C. Spivey 'Studies Towards the N-acylative Kinetic Resolution of NOBIN', Coll. Czech. Chem. Comm. 2011, 76 (Special Issue to Celebrate 60th Birthday of Pavel Kočovský), 1239-1253 [PDF(372KB)];
- A.C. Spivey, S. Arseniyadis, ‘Amine, Alcohol & Phosphine Catalysts for Acyl Transfer Reactions’, in Topics in Current Chemistry: Asymmetric Organocatalysis, Ed. B. List B., Springer Verlag,2008, Chapter 25 [PDF (3.02MB)];
- A.C. Spivey, S. Arseniyadis, T. Fekner, A. Maddaford, D.P. Leese, 'Rapid, room-temperature acylative kinetic resolution of sec-alcohols using atropisomeric 4-aminopyridine/triphenylphosphine catalysis' Tetrahedron 2006, 62, 295-301. [PDF (271KB)];
- A.C. Spivey, S. Arseniyadis, 'Nucleophilic catalysis by 4-(dialkylamino)pyridines revisited - the search for optimal reactivity and selectivity' Angew. Chem. Int. Ed. 2004, 43, 5436-5441. [PDF(378KB)]. see also: Publications
Ge-based phase-tagged synthesis
Flexible OFET with ultra thin aluminium oxide gate insulator
We are interested in exploiting some unique properties of germanium for organic synthesis. Specifically, we are developing iterative phase-tagged approaches for the preparation of oligomeric organic semiconductors for potential applications in plastic electronic devices.
We are developing new germanium (Ge) based linkers for the synthesis of aromatic and heteroaromatic compounds attached to phase tags. By phase tag we mean either an insoluble polymer [i.e. for Solid Phase Synthesis (SPS)] or a perfluorocarbon chain [i.e. for Fluorous phase Synthesis (FPS)]. Organogermanes have a number of interesting properties which we believe make them potentially very useful as constituents of linkers. Aryl germanes are particularly interesting in view of their high stability to basic conditions and their high susceptibility to aromatic ipso-substitution by electrophiles.
We have previously used these linkers for the preparation of conjugated heterocyclic oligomers with novel electro-optical properties as part of a wider collaborative project funded by the EPSRC Carbon Based Electronics initiative: The Organic Materials for Electronics Consortium (OMEC). Compounds of this type are of great current interest as components of plastic electronic devices.
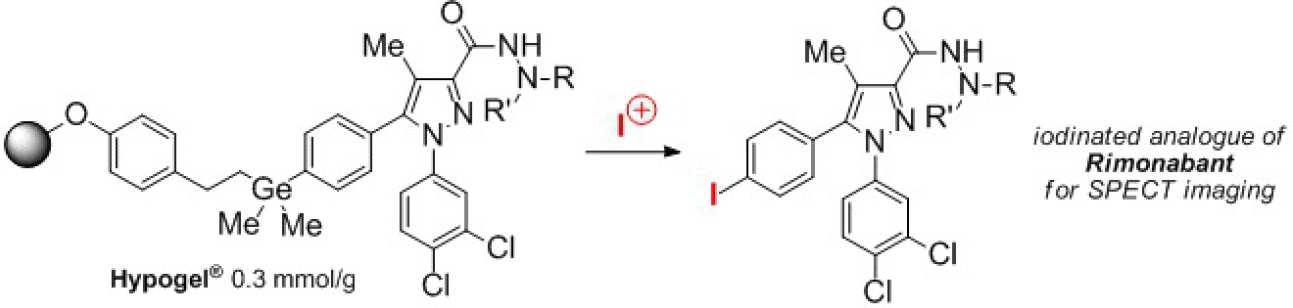
We are currently working on novel procedures for attachment of aromatic compounds to our germanium linker using palladium catalysed cross-coupling of di-metallo derivatives and also exploring conditions for transition metal catalysed cross-coupling as a method for cleavage from the resin with concomitant C-C bond formation. We are also exploring the use of these linkers for isotopic labeling for Positron Emission tomography (PET) and for pharmaceutical/agrochemical library preparation, e.g.
Our germ anium linkers are also commercially available from AF ChemPharm, Sheffield, UK.
Funded by
- BBSRC; EPSRC; Syngenta, Jealotts Hill; GE Healthcare (ex. Amersham Health); GSKStevenage; sanofi-aventis, Alnwick; Avecia, Blackley Manchester; Pfizer Vetenar y Medicines, Sandwich, CRUK
Collaborations with
-
Prof Eric Aboagye for PET Imaging relating to cancer (Imperial College London, department of Oncology, Hammersmith Campus, London, UK).
Selected Publications
- C.-C. Tseng, M. Li, B. Mo, S.A. Warren, A.C. Spivey, 'Stereocontrolled Formation of Styrenes by Pd(0)-Catalyzed Cross-Coupling of Photoactivated (E)-Alkenylgermanes with Aryl Bromides',Chem. Letts. 2011, Special Issue Focusing on the Mizoroki-Heck & Cross-coupling Reactions,40, 995-997 [PDF (904KB)];
- A.C. Spivey, C.-C. Tseng, T.C. Jones, A.D. Kohler, G.J. Ellames, 'A Method for the Parallel Solid-Phase Synthesis of Iodinated Analogues of the CB1 Receptor Inverse Agonist Rimonabant', Org. Lett. 2009, 11, 4760-4763. [PDF (295KB)];
- A.C. Spivey, L.J. Martin, C.-C. Tseng, G.J. Ellames, A.D. Kohler, 'A strategy for isotope containment during radiosynthesis - devolatilisation of bromobenzene by fluorous-tagging - Ir-catalysed borylation en route to the 4-phenylpiperidine pharmacophore' Org. Biomol. Chem. 2008, 6, 4093-4095 [PDF (141KB)];
- A.C. Spivey, L.J. Martin, C. Noban, T.C. Jones, G.J. Ellames, A.D. Kohler, 'Opportunities for isotopic labelling via phase-tagged synthesis with organogermanium linkers' J. Label. Compd. Radiopharm. 2007, 50, 281-285 [PDF (139KB)]. See also: Publications
Contact details
Email: a.c.spivey@imperial.ac.uk
Skype: alan.spivey
Tel & Fax: +44 (0)20 75945841
Further links: