 ATR-FTIR Spectroscopic imaging can be performed in combination with a number of custom designed cells which can be attached to the ATR crystal. These custom cells are each designed for a specific purpose and serve as "bolt on" accessories to an existing setup adding flexibility. The compaction cell is used in conjunction with a diamond ATR accessory and can be used to study in-situ compaction of tablets. The compaction and dissolution cell and Perspex cell are used for studying tablet dissolution.
ATR-FTIR Spectroscopic imaging can be performed in combination with a number of custom designed cells which can be attached to the ATR crystal. These custom cells are each designed for a specific purpose and serve as "bolt on" accessories to an existing setup adding flexibility. The compaction cell is used in conjunction with a diamond ATR accessory and can be used to study in-situ compaction of tablets. The compaction and dissolution cell and Perspex cell are used for studying tablet dissolution.
FTIR spectroscopic imaging overcomes the major limitation of the currently used USP dissolution tests, which are rather crude as they do not provide any insight into the physical and chemical means by which tablet dissolution proceeds. These latter two cells can importantly be used in conjunction with complementary analytical techniques; a UV/vis spectrometer can be connected online to produce a dissolution profile, while the Perspex dissolution cell can be used in conjunction with a standard video camera producing visible optical data. This use of complementary approaches is a very important part of performing a thorough analysis of the tablet's properties. The UV/vis profile data provide complementary information to that of the standard USP dissolution, while the video data has been used in combination with FTIR data in order to determine the position of dissolution fronts in swellable tablets .
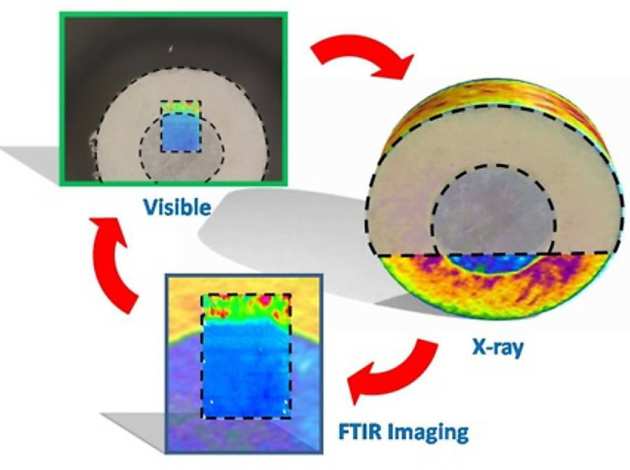
One feature of ATR imaging is that it obtains data from the bottom layer of the sample, therefore alongside these two supplementary in-situ analysis methods computed X-ray microtomography has been used. X-ray tomography provides a three dimensional density distribution of the sample and has been used for looking at tablet structure and the distribution of particles within a sample. It is important to use these approaches in a cooperative fashion in order to enhance the understanding of a system, as shown in the figure. Recently, we used combination of three spectroscopic imaging methods ATR-FTIR spectroscopic imaging, Magnetic Resonance Imaging (MRI) and Raman mapping to understand the mechanism of drug release from amorphous multicomponent solid dispersions (Punčochová K. et al. Pharm. Res. 2017 doi Open Access, see cover imaging below).

Key References
- Kazarian, S. G. and Chan, K. L. A., (2006), Sampling Approaches in Fourier Transform Infrared Imaging Applied to Polymers, Progress in Colloid and Polymer Science, 132(1), 1-6.
- Kazarian, S. G. and Chan, K. L. A., (2003), Chemical Photography Of Drug Release, Macromolecules, 36(26), 9866.
- Kazarian, S. G., Kong, K. W. T., Bajomo, M., Weerd, J. V. and Chan, K. L. A., (2005), Spectroscopic Imaging Applied to Drug Release, Food and Bioproducts Processing, 83(C2), 127-135.
- Chan, K. L. A., Elkhider, N., Kazarian, S. G., Spectroscopic imaging of compacted pharmaceutical tablets , Chem. Eng. Res. Des. (2005) Pages: 1303 - 1310, (doi)
- Chan, K. L. A., Fleming, O. S., Kazarian, S. G., et al, Polymorphism and devitrification of nifedipine under controlled humidity: a combined FT-Raman, IR and Raman microscopic investigation , J RAMAN SPECTROSC (2004) Vol: 35 , Pages: 353 - 359 , (doi)
- Kazarian S. G., Van der Weerd J. (2008) Simultaneous FTIR spectroscopic imaging and visible photography to monitor tablet dissolution and drug release Pharm. Res. 25, 853-860.
- Wray, P. S., Chan, K. L. A., Kimber, J. and Kazarian, S. G., (2008), Compaction of Pharmaceutical Tablets with Different Polymer Matrices Studied by FTIR Imaging and X-Ray Microtomography, Journal of Pharmaceutical Sciences, 97(10), 4269-4277.
- Kazarian S. G., Ewing A. V. (2013) Applications of Fourier transform infrared spectroscopic imaging to tablet dissolution and drug release Expert Option on Drug Delivery 10(9),1207-1221 (doi)
- Wray P. S., Li J., Li L. Q, Kazarian S. G. (2014) Combined Study of Biphasic and Zero Order Release with Dissolution Tests and ATR-FTIR Spectroscopic Imaging J. Pharm. Sci. 103 (7) 1995–2004 (doi)
- Ewing A.V., Clarke G. S., Kazarian S. G. (2014) Stability and Release of Indomethacin from Amorphous Solid Dispersions Studied with ATR-FTIR Spectroscopic Imaging European Journal of Pharmaceutical Sciences 60, 64-71.
- Punčochová K., Ewing A.V., Gajdošová M., Sarvašová N., Kazarian S. G., Beránek J., Štěpánek F. (2015) Identifying the mechanisms of drug release from amorphous solid dispersions using MRI and ATR-FTIR spectroscopic imaging International Journal of Pharmaceutics 483, 256-267. doi:10.1016/j.ijpharm.2015.02.035 )
- Punčochová K., Ewing A. V., Gajdošová M., Pekárek T., Beránek, J., Kazarian S. G., Stepanek F. (2017) The combined use of imaging approaches to assess drug release from mutlicomponent solid dispersions Pharmaceutical Research 34, 990-1001. (doi Open Access)