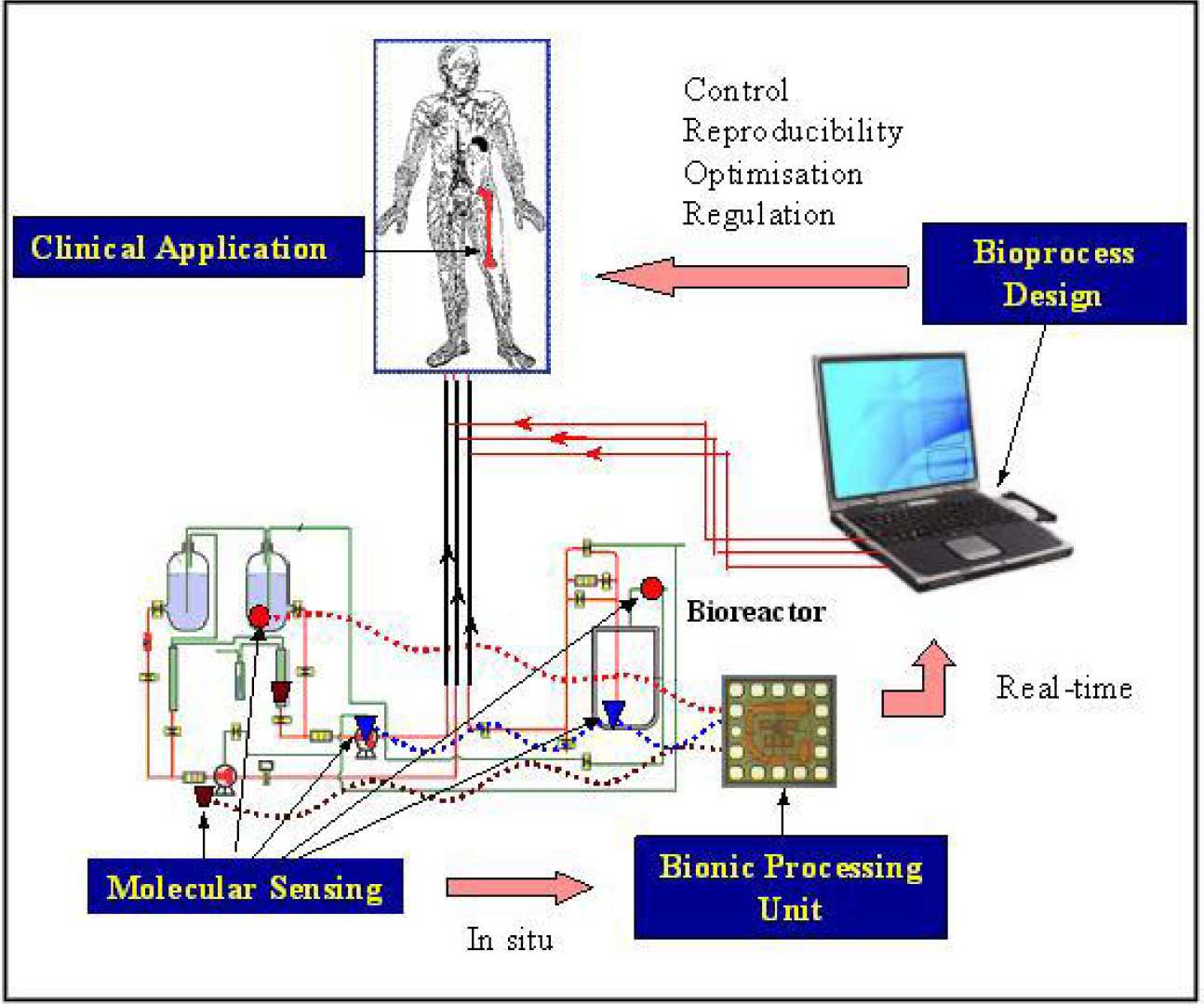
The fundamental bottleneck of any bioprocess is the lack of real-time, on-line, in-situ, quantitative information with respect to cellular behaviour in culture. As a result, control, optimisation, and scale-up of bioprocesses are essentially manual (empirical), which results in sub-optimal productivity (i.e., inadequate cell expansion) and product quality (i.e., inconsistent cell phenotypes). To harness the immense potential of stem cells (SCS) in terms of their plasticity and expansion capabilities, the physiological activity in relation to the culture parameters (local) such as pH, dissolved oxygen, nutrients/metabolite concentrations and growth factor concentrations needs to be recorded quantitatively with the needed level of accuracy and subsequently evaluated in a biologically meaningful manner. BSEL, in collaboration with Dr. Drakakis (Bioengineering), Profs Cass and Toumazou (IBE), and Prof. Polak (TERM) is seeking to develop such a novel monitoring modality that allows the systematic development of clinically relevant culture systems and methodologies, which control and regulate stem cell self-renewal, expansion, differentiation, and death. Ultimately, such a breakthrough will lead to the engineering of reproducible, well-characterised, regenerated “designer” tissues and organs that meet the strict regulatory criteria for clinical applications. The engineering challenge involved in the fabrication of the proposed modality can only be met by the cross-fertilisation and amalgamation of expertise of cell biologists, engineers, scientists, and clinicians.
Current projects
- Integrating a novel engineered process with a real-time biosensing platform for stem cell and tissue engineering applications
- Controlling mesodermal differentiation of embryonic stem cell and its application to bone and cartilage tissue engineering
- Production of pneumocytes from embryonic stem cells using bioreactor
- Developing a 3D, high-throughput screening system for embryonic stem cells
- Integrating embryonic stem cell culture using encapsulation of ES cells and culture in bioreactors for the production of bone
- Characterisation of a Conditioned Media for Enhanced Mesoderm Differentiation
- Intelligent Stem Cell Culture Systems
Miss Maya Lim
Cell culture studies such as ex-vivo expansion of haematopoietic stem cells are fundamentally complex in nature, even until today, process characteristics that control and determine the process remain incomplete. The desire to achieve a truly engineered population of cells with high yield and quality remains to be realised. Our goal in this project is to use design of experiments (DOE) and real-time monitoring to help us obtain a better understanding of the process and exert process control. DOE is a long-established methodology used in many industrial applications for process characterisation studies and process control. Its effectiveness in screening and characterisation techniques will enable use to determine and quantify culture parameters influencing our process. This information in turn can be used to optimise the process that is desired. The presence of a real-time monitoring platform will enable us to monitor process parameters closely. Any changes or disturbances to the process can be immediately detected allowing the experimenter to make necessary changes to the process. This capability allows us to exert tight process control which will ultimately give a highly reproducible and desirable set of cell population.
Mr. Yu-Shik Hwang
Although considerable progress to direct ES cell differentiation to specific mesodermal lineage has been made, before ES cells may be used as a good cell source in cell and tissue therapy, there are still remaining prerequisites which have to be fulfilled regarding ES cell differentiation: 1) more efficient method for increasing the differentiation efficiency to desired cell type together with reducing differentiation to undesired cell types from ES cell has to be established. 2) developing time and cost effective culture system for controlling ES cell differentiation is essential. Therefore, strategy of this research is to develop a culture system for enhancing and controlling lineage specific differentiation of ES cell, especially mesodermal differentiation, with much higher efficiency forward specific cell type differentiation, and is to apply this system to skeletal tissue engineering, bone and cartilage.
Dr. Siti Ismail
Understanding the physiology of the body’s tissues is the first step in the tissue engineering process. The goal of tissue engineering is to assist the body in producing a material that closely matches or mimics the native tissue. To create pneumocytes in vitro, the physiology of the tissue must be understood to determine its biological and chemical properties. Being in contact with the external environment, they are frequently injured and continuous proliferation of the alveolar epithelium is necessary to maintain cellular homeostasis. Type I and type II pneumocytes play an essential role in pulmonary physiology. Type II alveolar epithelial cells have been derived from murine embryonic stem cells, but the capacity to generate differentiated airway epithelial tissue has not been reported.
The study of the differentiated functions and proliferate capacity of type II cells in response to injury in vivo has been hindered by the complexity of the systemic response to injury. Our goal is to design a bioreactor that permits pneumocytes expansion and also to provide a suitable in vivo environment for blood gas exchange. We used human embryonic stem cells via formation of embryoid bodies to investigate the process of embryoid bodies’ agglomeration both in 2D and 3D suspension cultures. To control cell-cell interacti ons, the embryonic bodies were encapsulated in alginate beads. These embryoid bodies will then differentiate into pneumocytes with the use of a bioreactor under different culture medium. However, the complex pulmonary structures have complicated the study and design of a suitable bioreactor. This is the first attempt to design and study a cell-like reactor, similar to a pulmonary environment. Although several bioreactors related methodologies have been developed, there have not been any systematic studies to determine the engineering parameters necessary for the production of pulmonary cells.
The overall aim of the project is to develop of three-dimensional (3-D), high-throughput screening system for embryonic stem (ES) cell culture in order to investigate the effects of multiple culture parameters such as co-culture, nutrients, growth factors, and components of extracellular matrix (ECM), using bone differentiation as a model. Presently the differentiation of ES cells to cell types required for transplantation therapy is limited by the ill-defined process of cellular differentiation and the concomitant differentiation of cells to undesired types. High-throughput screening methods offer the potential to investigate the composite effect of many factors and elucidate their complex interactions. Furthermore, we intend to develop an integrated, 3-D culture process for ES cells that would allow for their easy culture with little operator input.
To achieve this, we propose to investigate the differentiation of single ES cells encapsulated in alginate, a biocompatible hydrogel isolated from seaweed. This offers the following advantages: (1) a physiologically more accurate 3-D culture microenvironment; (2) a means of incorporating molecules, such as a insoluble matrix pr oteins, growt h factors, etc. within the hydrogel, which can affect self-renewal or differentiation; (3) the ability to reproducibly grow a single cell; and (4) fact that each individual hydrogel bead represents an individual bioreactor allo wing for the si multaneous exami na tion of m ultiple culture parameters at different time points during the culture time.
Our preliminary data show that single ES cells can be encapsulated in alginate and grow and divide to form undifferentiated colonies. 23 of 41 alginate-encapsulated single cells divided to form compact colonies after 10 days. The colonies released from alginate stained strongly and uniformly for both CD9 and Oct4, demonstrating that these cells remain undifferentiated.
In this work, we intend to perfect the encapsulation techniques for single ES cells in order to develop a reproducible methodology. Furthermore, we intend to utilise single cell analysis techniques to assay and evaluate the effect of various factors on ES cell proliferation and differentiation. The development of this novel methodology may be used to facilitate high-throughput screening of many different differentiation protocols. It could also be used to determine the proliferative potential of single ES cells induced to differentiate along a specific lineage.
The transition from an undifferentiated mouse Embryonic Stem Cell (mESC) to bone tissue using traditional protocols in 2-D culture is fragmented, undefined, involves high maintenance, difficult to sample, and highly variable. Traditional Embryonic Stem Cell (ESC) culture in 2-D cultures involves three stages: a) ESC maintenance, b) Embryoid Body (EB) differentiation, and c) lineage-specific differentiation. Each stage requires manipulation and stage-specific protocols. The goal for this project is the integration of the various steps in ESC culture using bioreactors resulting in the reproducible, straightforward, high intensity culture of ESCs for clinical bone tissue engineering applications. The hypothesis is that encapsulation of ESCs in 3-D alginate beads would result in an environment that would be conducive for the maintenance of ESC, EB formation, and osteogenic differentiation. This will allow for automation, control, optimisation, and intensification of the process producing the clinically relevant numbers of osteogenic cells required in clinical applications. Furthermore, the use of alginate beads presents several advances because they are biocompatible, have FDA approval, are easy to dissolve, or can be used to inject directly into the patient with encapsulated cells.
Miss Yunyi Kang
The overall objective of this project is to search for an active signalling factor or factors present in Hep G2-conditioned medium and apply these factors to develop a chemically defined medium for an optimum mesoderm differentiation, which can be further applied to the bone and cartilage tissue engineering. Lineage-specific differentiation to osteoblasts and chondrocytes can be achieved through the mesoderm enhancement by manipulating culture conditions together with the application of those actively biological components. Therefore this study will allow us to develop a novel process of efficient lineage specified differentiation. This study can also be a preliminary step to untangle the complexity of signalling system to embryogenesis as well as signalling to differentiation of ES cells. The project approach will include proteomic analysis together with various bioassay techniques.
Dr. Cathy Ye
The fundamental bottleneck of any bioprocess is the lack of real-time, on-line, in-situ, quantitative information with respect to cellular behaviour in culture. As a result, control, optimisation, and scale-up of bioprocesses are essentially manual (empirical), which results in sub-optimal productivity (i.e., inadequate cell expansion) and product quality (i.e., inconsistent cell phenotypes). To harness the immense potential of stem cells (SCs) in terms of their plasticity and expansion capabilities, the physiological activity in relation to the culture parameters (local) such as pH, dissolved oxygen, nutrients/metabolite concentrations and growth factor concentrations needs to be recorded quantitatively with the needed level of accuracy and subsequently evaluated in a biologically meaningful manner. We propose the development of such a novel monitoring modality that allows the systematic development of clinically relevant culture systems and methodologies, which control and regulate stem cell self-renewal, expansion, differentiation, and death. Ultimately, such a breakthrough will lead to the engineering of reproducible, well-characterised, regenerated “designer” tissues and organs that meet the strict regulatory criteria for clinical applications. The engineering challenge involved in the fabrication of the proposed modality can only be met by the cross-fertilisation and amalgamation of expertise of cell biologists, engineers, scientists, and clinicians.
Haematopoietic culture systems have been selected as the test case based on their clinical significance, tangible clinical applications, and the wealth of culture data for both adult and embryonic stem cells, which will serve as a measure for comparison. Haematopoiesis is a highly dynamic process regulated and controlled in vivo by the haematopoietic microenvironment and encompasses parameters such as pH, local oxygen concentration, the composition and concentration of growth factors, the supply of nutrients, and the cellular and molecular surrounding of the cells. Defining optimal culture parameter values for ex vivo expansion of haematopoietic stem and progenitor cells is challenging mainly due to the widely distributed - in terms of degree of differentiation cell population - especially since most studies ignore the complex kinetics, transient nature, intricate interactions between parameters, and the lack of invariant measures. Even slight deviations in the culture parameters can affect the final cell output.
The ove rall aim of this project is the design and evaluation of optimal, controlled, and reproducible culture systems and protocols for the expansion and differentiation of cord blood stem cells to satisfy the clinical bone marrow transplantation requirements.
Contact details
Professor Sakis Mantalaris
Head of BSEL Group
Chemical Engineering Department
Tel: +44 (0)20 7594 5601
Email:
a.mantalaris@imperial.ac.uk