Older publications are listed below - click here to view the most recent publications from the group.
48. The Acetate Proton Shuttle between Mutually trans Ligands
De Aguirre, A.; Díez-González, S.; Maseras, F.; Martín, M.; Sola, E. 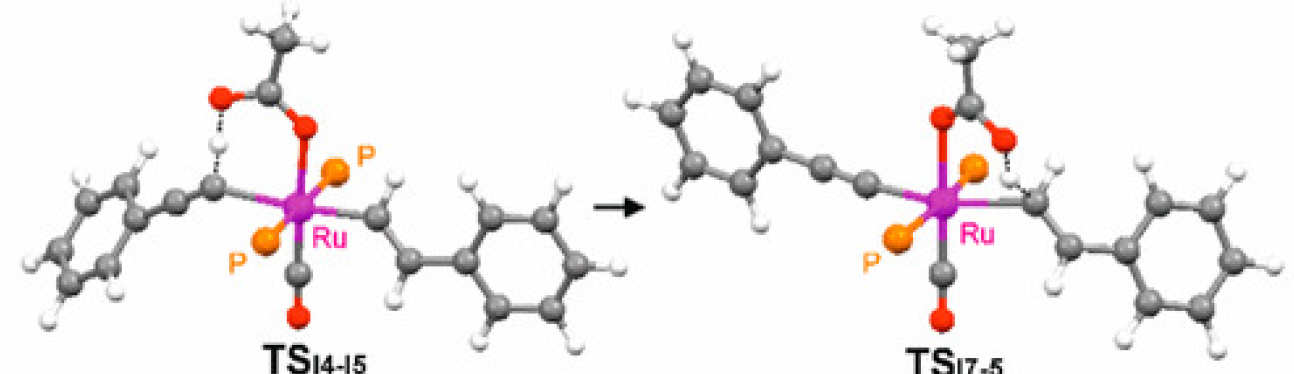
Organometallics, 2018, 37, 2645–2651
This work addresses a counterintuitive observation in the reactivity of the well-known ruthenium complexes [Ru(X)H(CO)(PiPr3)2], according to which the 5-coordinate chloro complex (X = Cl, 1) is less reactive toward phenylacetylene than its 6-coordinate acetate analogue (X = κO2-OC(O)Me, 3), since 3 undergoes a hydride-to-alkenyl-to-alkynyl transformation, whereas the reaction of 1 stops at the alkenyl derivative. The experimental kinetics of the key alkenyl-to-alkynyl step in the acetate complex are compared to the results of DFT calculations, which disclose the ability of the acetate not only to assist the alkyne C–H activation step via a CMD mechanism but also to subsequently deliver the proton to the alkenyl ligand. Possible consequences of this mechanistic resource connecting mutually trans ligands are briefly discussed on the basis of reported chemoselectivity changes induced by carboxylate ligands in 1-alkyne hydrosilylations catalyzed by this type of ruthenium complexes.
47. Thermal Azide-Alkene Cycloaddition Reactions: Straightforward Multi-gram Access to Δ2-1,2,3-Triazolines in Deep Eutectic Solvents
Sebest, F.; Casarrubios, L.; Rzepa, H. S.; White, A. J. P.; Díez-González, S.
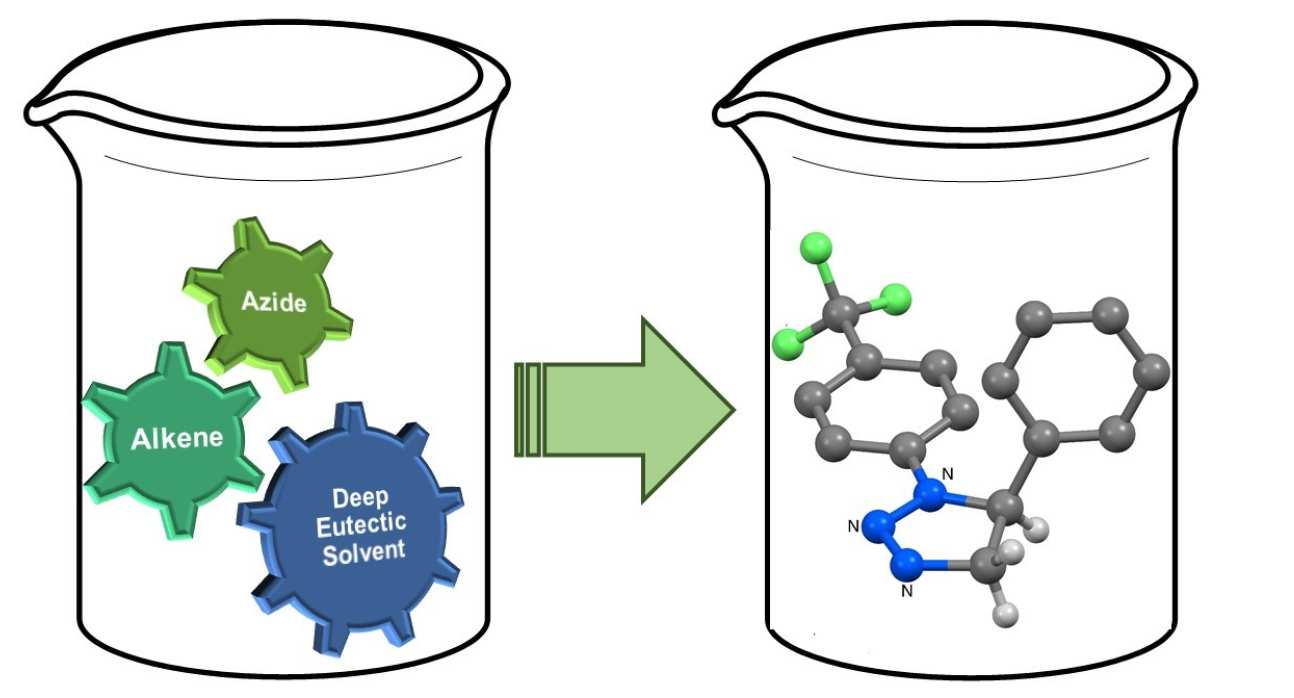
Green Chem. 2018, 20, 4023–4035
The multi-gram synthesis of a wide range of 1,2,3-triazolines via azide–alkene cycloaddition reactions in a Deep Eutectic Solvent (DES) is reported. The role of DES in this transformation as well as the origin of the full product distribution was studied with an experimental/computational-DFT approach.
46. Ring-expanded N-heterocyclic carbenes for copper-mediated azide-alkyne Click cycloaddition reactions
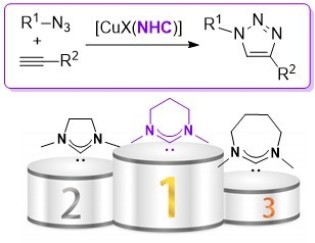
Sebest, F.; Dunsford, J. J.; Adams, M.; Pivot, J.; Newman, P. D.; Díez-González, S.
ChemCatChem 2018, 10, 2041-2045
In this article we studied different copper(I) complexes bearing ring‐expanded N‐heterocyclic carbene ligands in the azide–alkyne cycloaddition reaction. We showed that the six‐membered NHC ligands outperform well‐established five‐membered ones and [CuI(Mes‐6)] displayed a remarkable catalytic activity while respecting the strict criteria for click reactions.
45. Homo- and heteroleptic copper(I) complexes with diazabutadiene ligands: Synthesis, solution- and solid-state structural studies
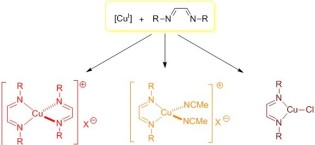
Zelenay, B.; Frutos-Pedreño, R.; Markalain-Barta, J.; Vega-Isa, E.; White, A. J. P.; Díez-González, S.
Eur. J. Inorg. Chem. 2016, 4649–4658
Herein we report the synthesis of several complexes with diazabutadiene complexes: [Cu(DABR)2]BF4), [Cu(DABR)(NCMe)2]BF4 and [CuCl(DABR)]. These complexes, which remain scarce in the literature are air-stable and their behaviour both in the solid state as well as in solution was studied by means of X‐ray crystallography, NMR and UV/Vis spectroscopy.
44. Copper(I)-acetylides: Access, structure and relevance in catalysis
Díez-González, S.
Advances in Organometallic Chemistry, 2016, Vol 66, 93-141
Pérez, P. J., Ed; Academic Press
43. Functionalised [(NHC)Pd(allyl)Cl] complexes: Synthesis, immobilisation and application in cross-coupling and dehalogenation reactions
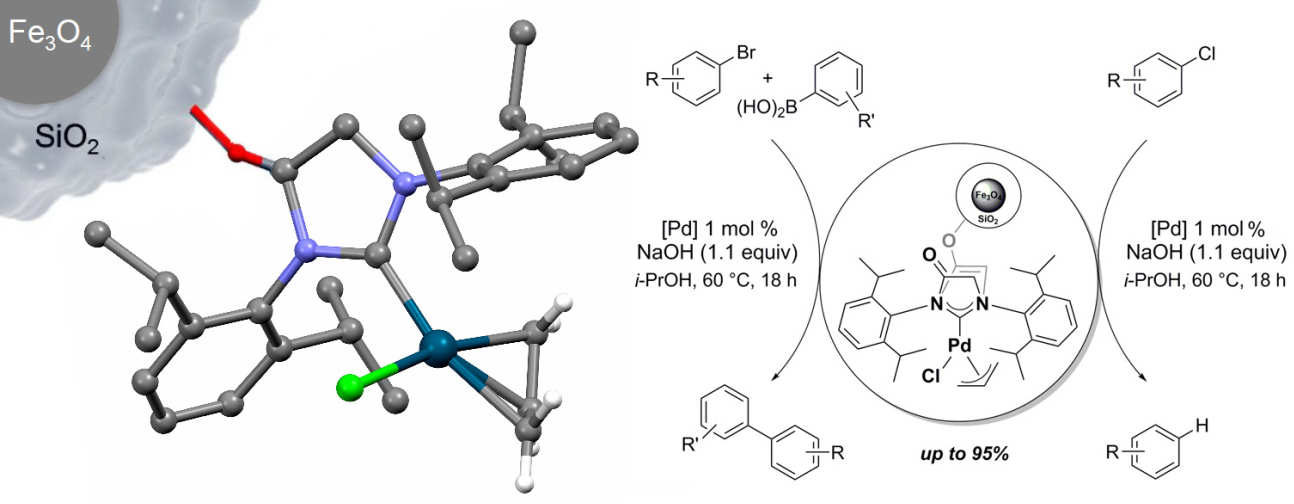
Collinson, J.-M.; Wilton-Ely, J. D. E. T.; Díez-González, S.
Catal. Commun. 2016, 87, 78–81
A novel NHC–palladium(II) complex and its immobilised version were prepared and fapplied in Suzuki-Miyaura cross coupling and chloroarene dehalogenation reactions. Also, an unexpected palladium-mediated transfer hydrogenation of a carbonyl compound was evidenced.
42. Copper(I)‒phosphinite complexes in Click chemistry : Three component reactions and preparation of 5-iodotriazoles
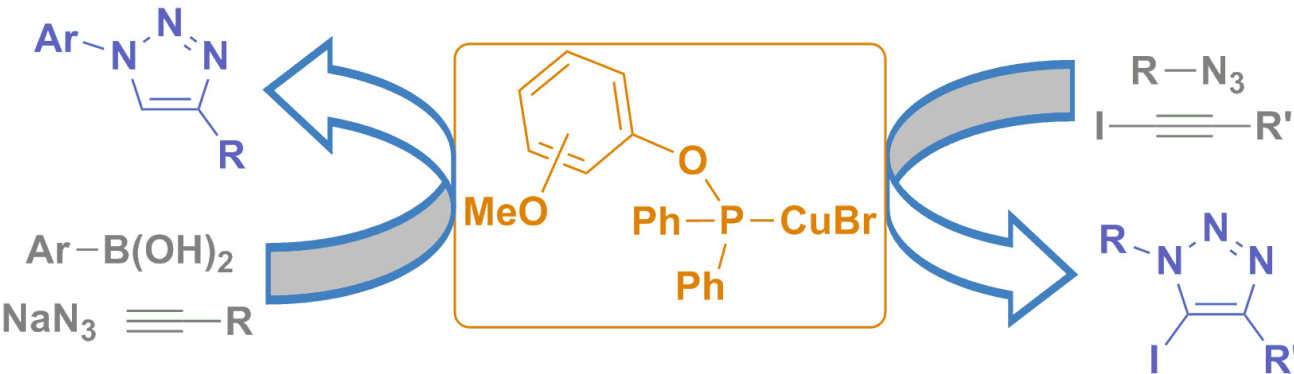
Pérez, J. M.; Crosbie, P.; Lal, S.; Díez-González, S.
ChemCatChem 2016, 8, 2222–2226
The remarkable activity displayed by copper(I)–phosphinite complexes of general formula [CuBr(L)] in two challenging cycloadditions is reported: a) the one‐pot azidonation/cycloaddition of boronic acids, NaN3, and terminal alkynes; b) the cycloaddition of azides and iodoalkynes. These air‐stable catalysts led to very good results in both cases and the expected triazoles could be isolated in pure form under ‘Click‐suitable’ conditions.
41. HBF4-Catalysed nucleophilic substitutions of propargylic alcohols
Barreiro, E.; Sanz-Vidal, A.; Tan, E.; Lau, S.-H.; Sheppard, T. D.; Díez-González, S.
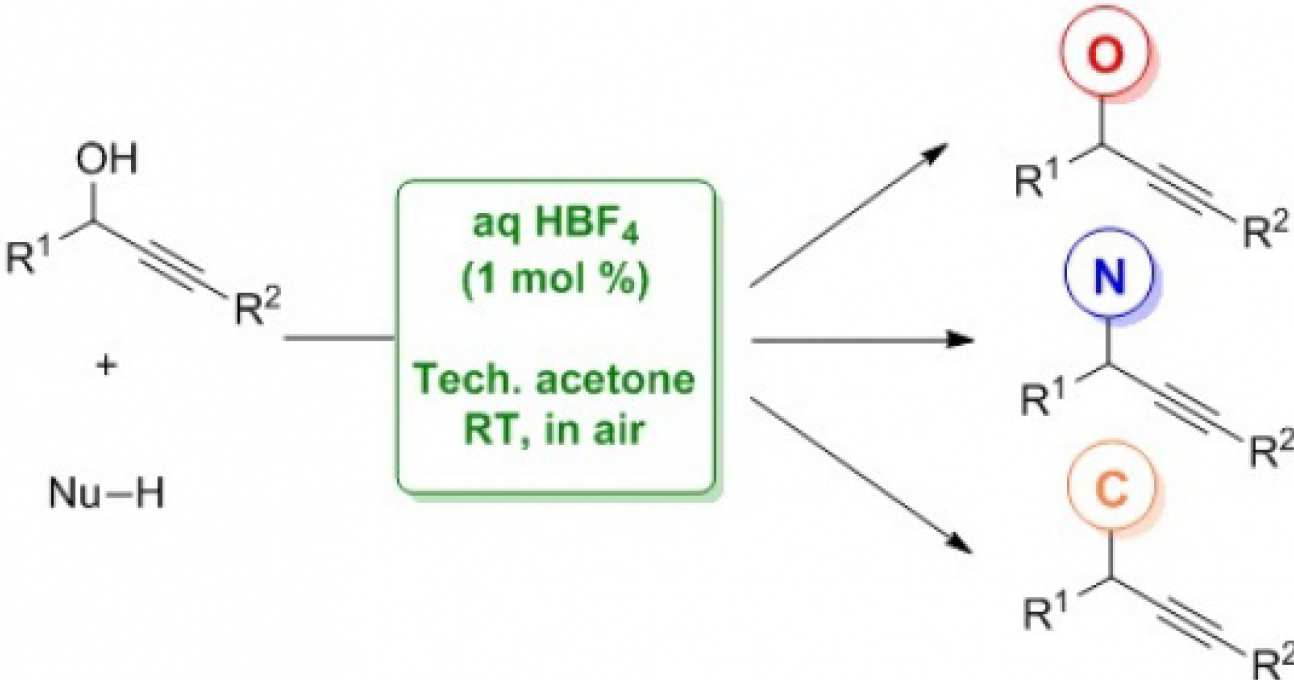
Eur. J. Org. Chem. 2015, 7544-7549
The activity of HBF4 (aqueous solution) as a catalyst in propargylation reactions is presented. Diverse types of nucleophiles were employed in order to form new C–O, C–N and C–C bonds in technical acetone and in air. Good to excellent yields and good chemoselectivities were obtained using low acid loading (typically 1 mol‐%) under simple reaction conditions.
40. Catalytic and mechanistic studies of N-heterocyclic carbene or phosphine-containing copper(I) complexes for the synthesis of 5-iodo-1,2,3-triazoles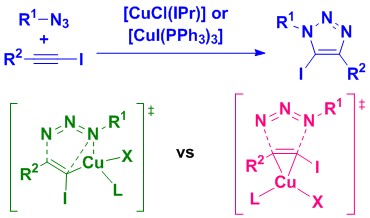
Lal, S.; Rzepa, H. S.; Díez-González, S.
ACS Catalysis 2014, 4, 2274-2287
Two complementary catalytic systems are reported for the 1,3-dipolar cycloaddition of azides and iodoalkynes. These are based on two commercially available/readily available copper complexes, [CuCl(IPr)] or [CuI(PPh3)3], which are active at low metal loadings (PPh3 system) or in the absence of any other additive (IPr system). These systems were used for the first reported mechanistic studies on this particular reaction. An experimental/computational-DFT approach allowed to establish that (1) some iodoalkynes might be prone to dehalogenation under copper catalysis conditions and, more importantly, (2) two distinct mechanistic pathways are likely to be competitive with these catalysts, either through a copper(III) metallacycle or via direct π-activation of the starting iodoalkyne.
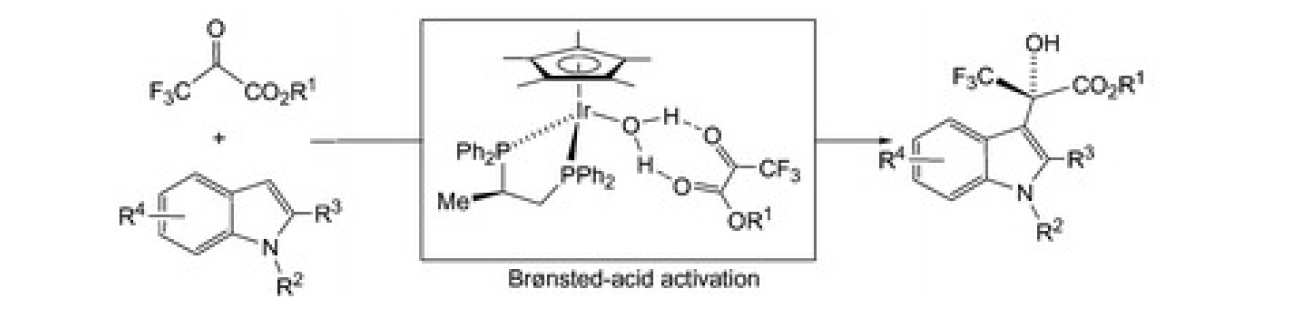
39. Chiral transition-metal complexes as Brønsted-acid catalysts for the asymmetric Friedel-Crafts hydroalkoxylation of indoles
Carmona, D.; Lamata, M. P.; Sánchez, A. Viguri, F.; Rodríguez, R.; Oro, L. A.; Liu, C.; Díez-González, S.; Maseras, F.
Dalton Trans. 2014, 43, 11260-11268
The Friedel–Crafts reaction between 3,3,3-trifluoropyruvates and indoles is efficiently catalysed by the iridium complex [(η5-C5Me5)Ir{(R)-Prophos}(H2O)][SbF6]2 (1) with up to 84% ee. Experimental data and theoretical calculations support a mechanism involving the Brønsted-acid activation of the pyruvate carbonyl by the protons of the coordinated water molecule in 1. Water is not dissociated during the process and, therefore, the catalytic reaction occurs with no direct interaction between the substrates and the metal.
This article is part of the themed collection 'Synergy between experimental and theory'

38. Reusable and highly active supported copper(I)–NHC catalysts for Click chemistry
Collinson, J.-M.; Wilton-Ely, J. D. E. T.; Díez-González, S. 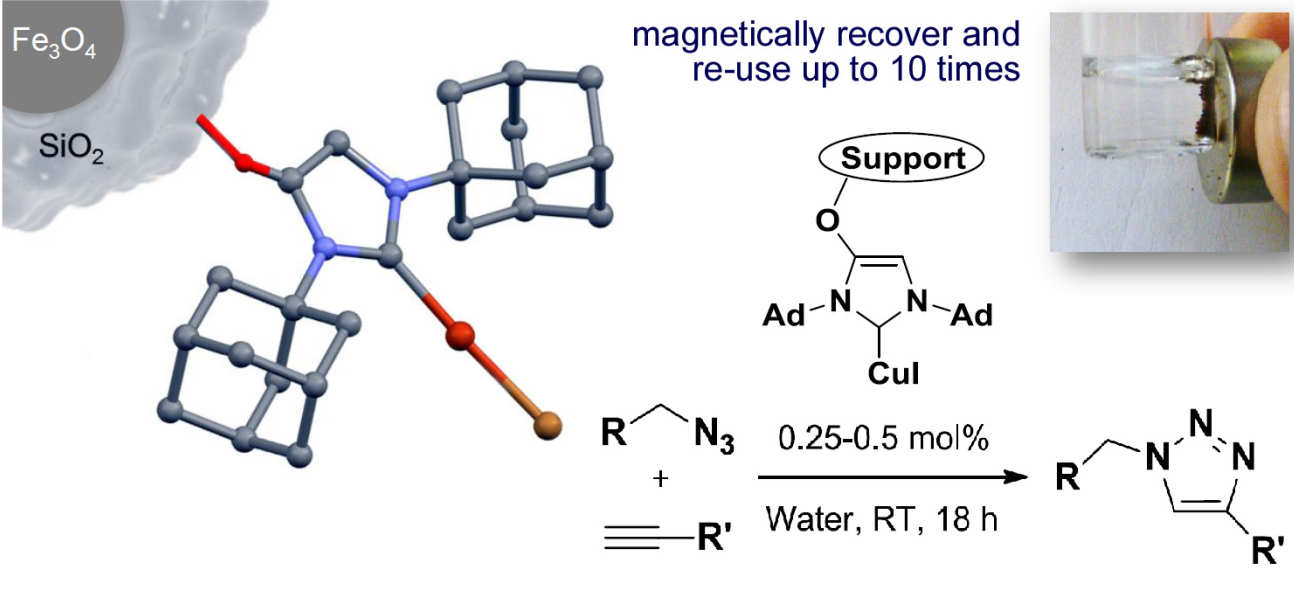
Chem. Commun. 2013, 49, 11358-11360
Immobilised [Cu(NHC)] catalysts are reported for the preparation of 1,2,3-triazoles. In addition to showing outstanding catalytic activity, the catalyst systems are easy to prepare and can be recycled many times.
This article is highligted in the back cover of the issue:
![Immobilised [Cu(NHC)] catalysts are reported for the Click preparation of 1,2,3-triazoles; these are highly active, easy to prepare and to recycle.](/media/migration/research-groups/8---2--tojpeg_1457694697950_x2.jpg)
37. Well-defined diimine copper(I) complexes as catalysts in Click azide-alkyne cycloaddition reactions
Markalain-Barta, J.; Díez-González, S.
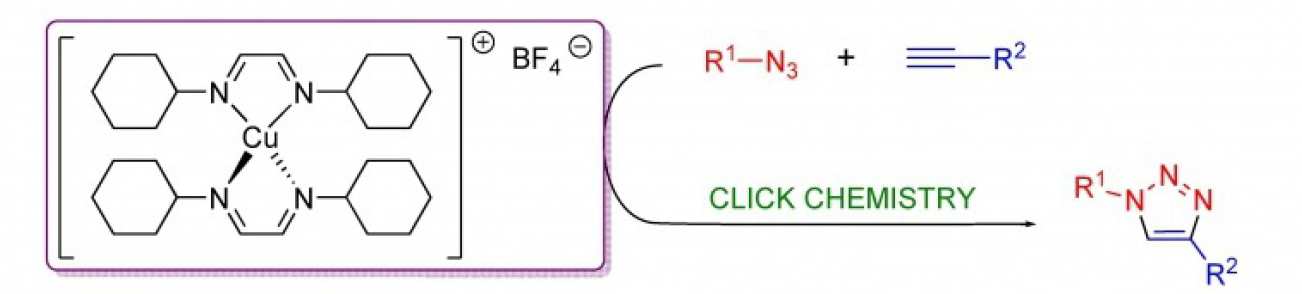
[Invited contribution to special issue on 'Advances in Click Chemistry']
A series of 1,4-disubstituted 1,2,3-triazoles have been prepared in high yields while respecting the stringent Click criteria. In these reactions, highly stable pre-formed complexes bearing diimine ligands were used.
36. On the unique reactivity of Pd(OAc)2 with organic azides: Expedient synthesis of nitriles and imines
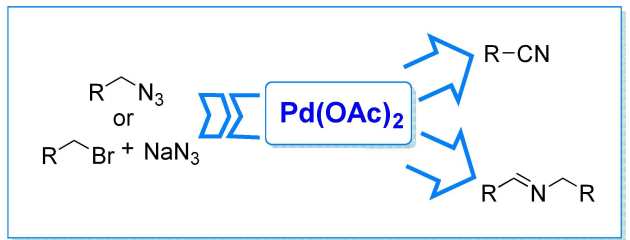
Martínez-Sarti, L.; Díez-González, S.
ChemCatChem 2013, 5, 1722–1724
Pd(OAc)2 can oxidise primary azides into their corresponding nitriles or imines under simple, technical conditions. This very user-friendly system also displays complementary selectivity to that of previously reported systems.
35. Synthesis of 1,3-bis(2,4,6-trimethylphenyl)imidazolium salts: SIMes∙HBr, SIMeS∙HBF4 and SIMes∙HPF6
Gautier, A.; Cisnetti, F.; Díez-González, S.; Gibard C.
Protocols Exchange 2012, doi:10.1038/protex.2012.048
N,N’–bis–(2,4,6–trimethylphenylamino)ethane dihydrobromide is obtained in a single step through the dialkylation of dibromoethane. It serves as a versatile starting material for the synthesis of imidazolium salts: SIMes.HBr, SIMes.HCl, SIMes.HPF6 and SIMes.HBF4.
34. Novel phosphinite and phosphonite copper(I) complexes: Efficient catalysts for Click azide-alkyne cycloaddition reactions
Lal, S.; McNally, J.; White, A. J. P.; Díez-González, S.
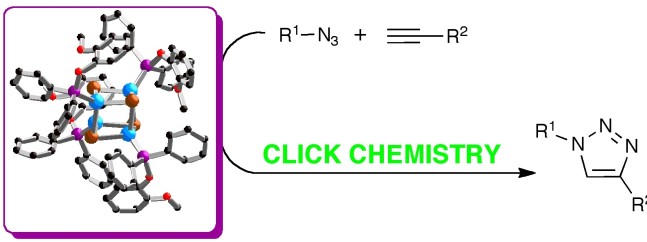
Organometallics 2011, 30, 6225–6232
In this article, the preparation of novel phosphinite- and phosphonite-bearing copper(I) complexes of the general formula [CuX(L)] is reported.
When compared to well-establised phosphine and phosphite analogues, these compounds displayed improved catalytic activity in the 1,3-dipolar cycloaddition of azides and alkynes. Full optimization of the reaction conditions resulted in a noteworthy Click catalytic system, active under very mild reaction conditions in the absence of any additive and using low metal loadings.
33. Well-defined copper(I) complexes for Click azide-alkyne cycloaddition reactions: One Click beyond
Díez-González, S.
Catal. Sci. Technol., 2011, 1, 166–178
[Hot Article; Invited contribution]
This review focus on the importance/interest of using ligands and pre-formed complexes in the copper-catalysed azide-alkyne cycloaddition reactions. Coordinated copper(I) species are more stable and tend to display an excellent catalytic efficiency. Furthermore, ligand can also modulate the actual activity and change the product distribution in some cases.
This Perspective was featured in the inside front cover.
32. The use of ligands in copper-catalyzed [3+2] azide-alkyne cycloaddition: Clicker than Click chemistry?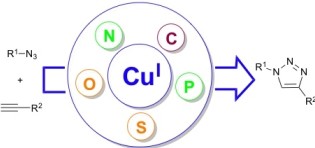
Díez-González, S.
31. [CuBr(PPh3)3] for azide alkyne cycloaddition reactions under true Click conditions
Lal, S.; Díez-González, S. 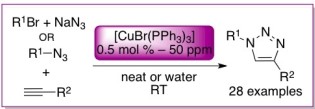
J. Org. Chem. 2011, 76, 2367–2374
A careful methodological study revealed a true Click catalytic system based on commercially available [CuBr(PPh3)3]. This system is active at room temperature, with 0.5 mol % [Cu] (or less), in the absence of any additive, and it does not require any purification step to isolate pure triazoles.
30. N-Heterocyclic carbenes in copper-catalyzed reactions
Díez-González, S.
Book chapter in a volume written by 'some of the most talented young chemists in Europe'. This book provides an account of the latest research results in European chemistry based on a selection of leading young scientists participating in the 2008 European Young Chemists Award competition.
Ideas in Chemistry and Molecular Science, Vol. 1; Advances in Synthetic Chemistry, Wiley-VCH, 2010 pp 43–66.
Earlier Publications
29. Díez-González, S.; Escudero-Adán, E. C.; Benet-Buchholz, J.; Stevens, E. D.; Slawin, A. M. Z.; Nolan, S. P. ‘[(NHC)CuX] complexes: Synthesis, characterization and catalytic studies’ Dalton Trans. 2010, 39, 7595–7606.
28. Ramón, R. S.; Bosson, J.; Díez-González, S.; Marion, N.; Nolan, S. P. ‘Au/Ag-cocatalyzed Beckmann rearrangement of aldoximes into primary amides’ J. Org. Chem. 2010, 75, 1197–120 2.
27. Díez-González, S.; Marion, N.; Nolan , S . P. ‘N-Heterocyclic carbenes in late-transition metals catalysis’ Chem. Rev. 2009, 1 09, 3612–3676.
26. Broggi, J.; Joubert, N.; Díez-González, S.; Berteina-Raboin, S.; Zevaco, T.; Nolan, S. P.; Agrofoglio, L. A. ‘Synthesis of (±)-1,2,3-Triazolo-4’,4-dihydroxymethyl-carbanuclesides’ Tetrahedron 2009, 65, 1162–1170.
25. Teyssot, M.-L.; Jarrousse, A.-S.; Chevry, A.; De Haze, A.; Beaudoin, C.; Manin , M.; Nolan, S. P.; Díez-González, S.; Morel, L.; Gautier, A. ‘Toxicity of copper(I)–NHC against human tumor cells: Induction of cell cycle arrest, apoptosis and DNA cleavage’ Chem.-Eur. J. 2009, 15, 314–318.
24. Díez-González, S.; Nolan, S. P. ‘[(NHC)2Cu]X complexes as efficient catalysts for azide-alkyne Click chemistry at low catalyst loadings' Angew. Chem., Int. Ed. 2008, 47, 8881–8884.
23. Díez-González, S.; Stevens, E. D.; Nolan, S. P. ‘A [(NHC)CuCl] complex as latent Click catalyst’ Chem. Commun. 2008, 4747–4749.
22. Díez-González, S.; Nolan, S. P. ‘N-Heterocyclic carbene copper complexes: Synthesis and catalysis’ Aldrichimica Acta 2008, 41, 43–51.
21. Díez-González, S.; Paugam, R.; Blanco, L. ‘Synthesis of 1-silabicyclo[4.4.0]dec-5-en-4-ones: A model of A and B rings of 10-silatestosterone’ Eur. J. Org. Chem. 2008, 3298–3307.
20. Díez-González, S.; Blanco, L. ‘Synthesis of 2-methylidene-1-silacyclohexanes by intramolecular hydrosilylation’ J. Organomet. Chem. 2008, 693, 2033–2040.
19. Díez-González, S.; Nolan, S. P. ‘Copper, silver and gold complexes in hydrosilylation reactions’ Acc. Chem. Res. 2008, 41, 349–358.
18. Broggi, J.; Díez-González, S.; Petersen, J. L.; Berteina-Raboin, S.; Nolan, S. P.; Agrofoglio L. A. ‘Study of copper(I) catalysts for the synthesis of carbanucleosides via azide-alkyne 1,3-dipolar cycloaddition’ Synthesis 2008, 141–148 [Feature article].
17. Díez-González, S.; Stevens, E. D., Scott, N. M.; Petersen, J. L.; Nolan, S. P. ‘Synthesis and characterization of [Cu(NHC)2]X complexes: Catalytic and mechanistic studies of hydrosilylation reactions’ Chem.−Eur. J. 2008, 14, 158–168.
16. Díez-González, S.; Nolan, S. P. ‘Transition metal-catalyzed hydrosilylation of carbonyl compounds and imines. A review’ Org. Prep. Proc. Int. 2007, 39, 523–560.
15. Broggi, J.; Joubert, N.; Aucagne, V.; Berteina-Raboin, S.; Díez-González, S .; Nolan, S.: Topalis, D.; Deville-Bonne, D.; Balzarini, J.; Neyts, J.; Andrei, G.; Snoeck, R.; Agrofoglio, L. A. ‘Alkyne-azide click chemistry mediated carbanucleosides synthesis’ Nucleosides, nucleotides and nucleic acids 2007, 26, 1391–1394.
14. Díez-González, S.; Nolan, S. P. ‘[(NHC)Copper(I)] complexes in homogeneous catalysis' Synlett 2007, 2158–2167.
13. Marion, N.; Díez-González S.; Nolan, S. P. ‘N-Heterocyclic carbenes as organocatalysts’ Angew. Chem., Int. Ed. 2007, 46, 2988–3000.
12. Lebel, H.; Davi, M.; Díez-González, S.; Nolan, S. P. ‘Copper-carbene complexes as catalysts in the synthesis of functionalized styrenes and aliphatic alkenes’ J. Org. Chem. 2007, 72, 144–149.
11. Díez-González S.; Nolan, S. P. ‘Stereoelectronic parameters associated with N-heterocyclic carbene (NHC) ligands: A quest for understanding’ Cood. Chem. Rev. 2007, 251, 874–883.
10. Díez-González, S.; Nolan, S. P. ‘Palladium-catalyzed reactions using NHC ligands’ N-Heterocyclic Carbenes in Transition Metal Catalysis: Top. Organomet. Chem. 2007, 21, 47–82.
9. Welle, A.; Díez-González, S.; Tinant, B.; Nolan, S. P.; Riant, O. ‘A three-component tandem reductive aldol reaction catalyzed by N-heterocyclic carbene-copper complexes’ Org. Lett. 2006, 8, 6059–6062.
8. Díez-González, S.; Correa, A.; Cavallo, L.; Nolan, S. P. ‘(NHC)Copper(I)-catalyzed [3+2] cycloaddition of azides and mono- o r disubstituted alkynes’ Chem.−Eur. J. 2006, 12, 7558–7564 [Higlighted in Synfacts 2007 (2) 142].
7. Díez-González, S.; Blanco, L. ‘Synthesis of 2-methylidene-1-silacyclohexanes from 2,6-dibromohex-1-ene and polyhalosilanes’ J. Organomet. Chem. 2006, 691, 5531–5539.
6. Marion, N.; Díez-González, S.; de Frémont, P.; Noble, A. R.; Nolan, S. P. ‘AuI-Catalyzed tandem [3,3] rearrangement-intramolecular hydroarylation: Mild and efficient formation of substituted indenes’ Angew. Chem., Int. Ed. 2006, 45, 3647–3650 [Hot paper].
5. Díez-González, S.; Scott, N. M.; Nolan, S. P. ‘Cationic copper(I) complexes as efficient pre-catalysts for the hydrosilylation of carbonyl compounds’ Organometallics 2006, 25, 2355–2358.
4. Díez-González, S.; Nolan, S. P. ‘Palladium pre-catalysts for cross-coupling reactions’ Spec. Chem. Mag. 2006, Jan/Feb, 26–28.
3. Díez-González, S.; Nolan, S. P. ‘Carbene and transition metal-mediated transformations’ Ann. Rep. Prog. Chem., Sect. B 2005, 101, 171–191.
2. Kelly, R. A., III; Scott, N. M.; Díez-González, S.; Stevens, E. D.; Nolan, S. P. ‘Simple synthesis of CpNi(NHC)Cl complexes (Cp = cyclopentadienyl ; NHC = N-heterocyclic carbene)’ Organometallics 2005, 24, 3442–3447.
1. Díez-González, S.; Kaur, H.; Zinn, F. K.; Stevens, E. D.; Nolan, S. P. ‘A simple and efficient copper-catalyzed procedure for the hydrosilylation of hindered and functionalized ketones’ J. Org. Chem. 2005, 70, 4784–4796.