Read summaries of selected recent publications from members of the Immuno-Pathology Network below.
Accordion widget
- Glycosylated nanoparticles derived from RAFT polymerization for effective drug delivery to macrophages
- A Probe for NLRP3 Inflammasome Inhibitor MCC950 Identifies Carbonic Anhydrase 2 as a Novel Target
- A TLR7 antagonist restricts interferon-dependent and -independent immunopathology in a mouse model of severe influenza
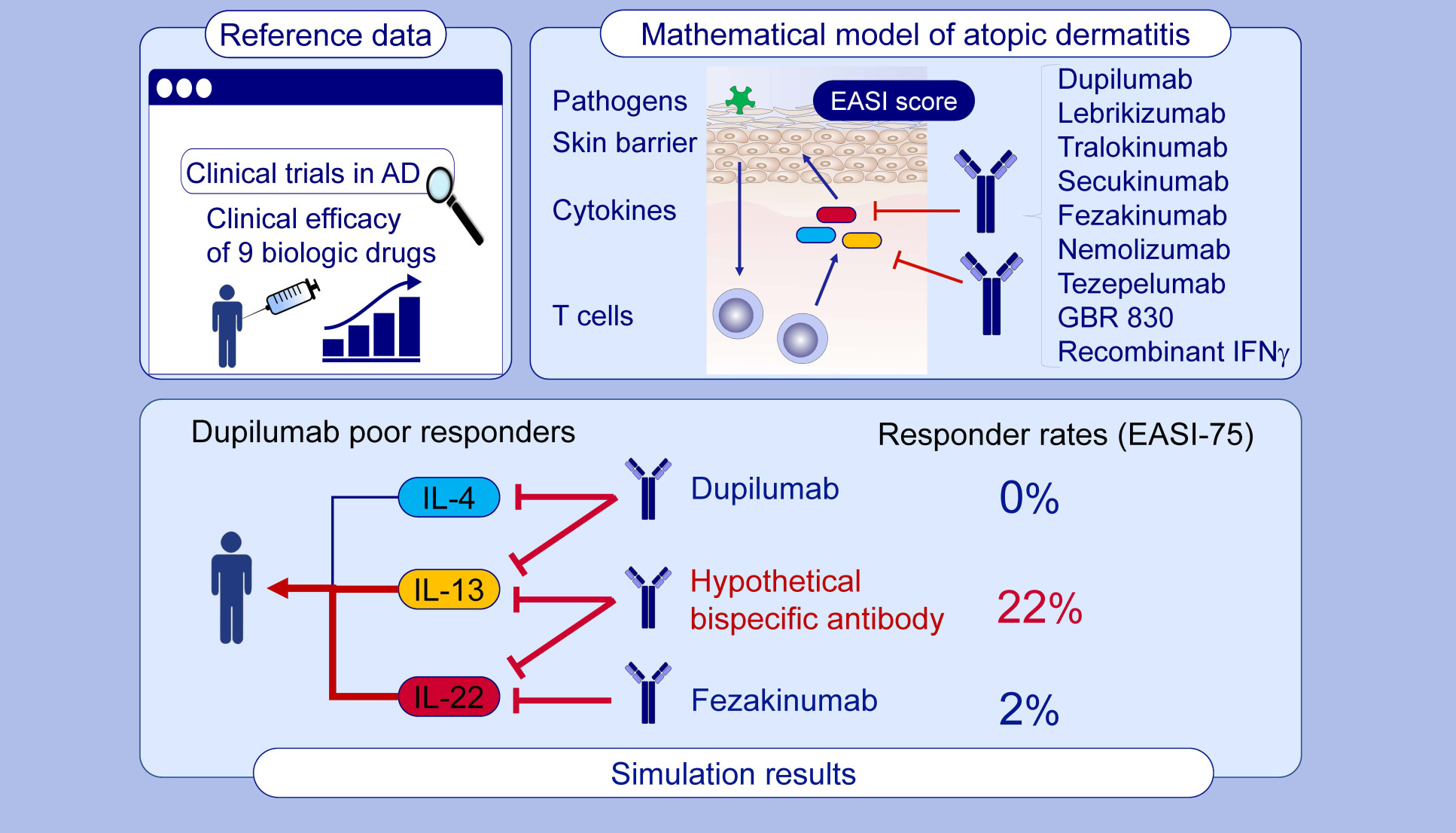
- A mathematical model to identify optimal combinations of drug targets for dupilumab poor responders in atopic dermatitis
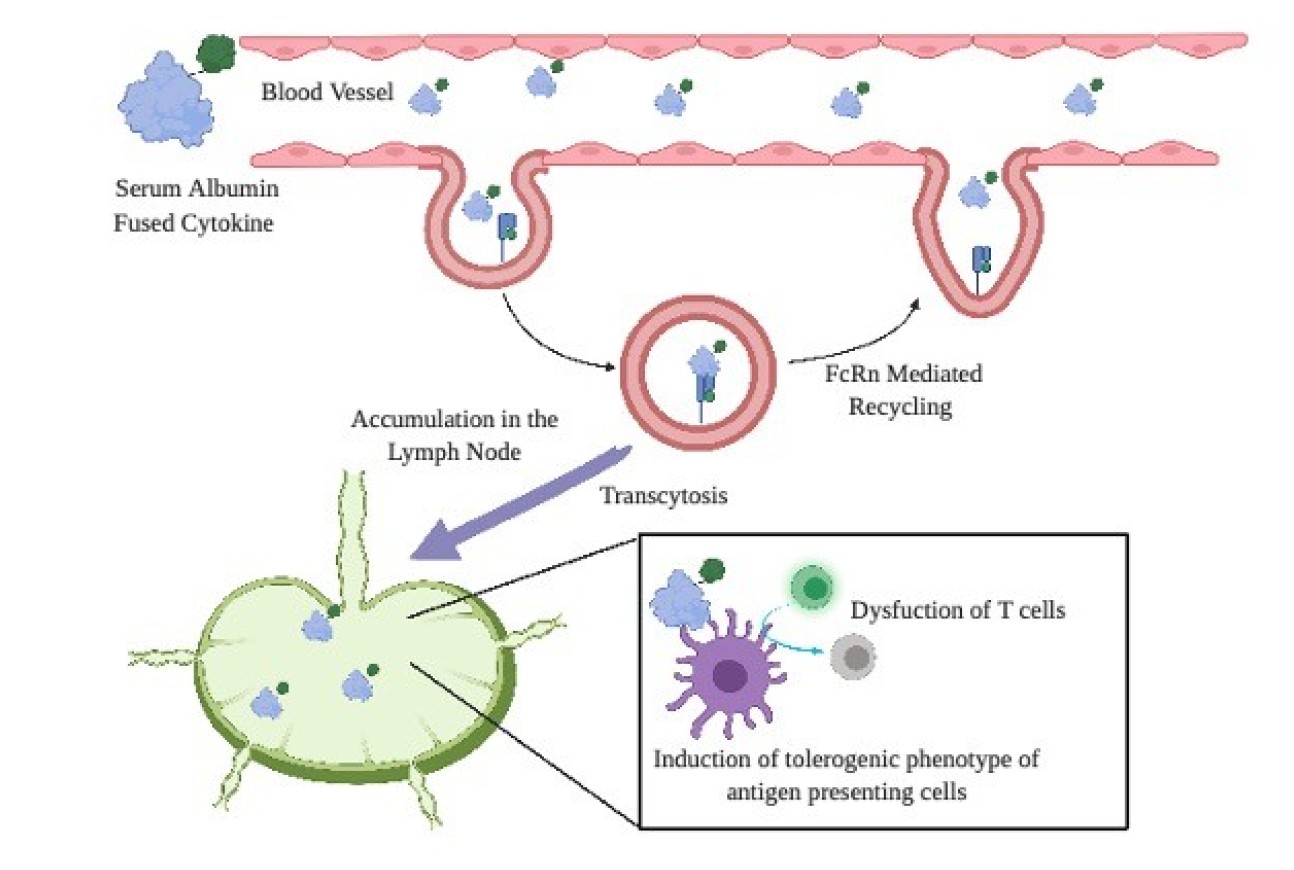
- Prolonged residence of an albumin–IL-4 fusion protein in secondary lymphoid organs ameliorates experimental autoimmune encephalomyelitis
- Application of direct stochastic optical reconstruction microscopy (dSTORM) to the histological analysis of human glomerular disease
- Autologous Hematopoietic Stem Cell Transplantation in Active Multiple Sclerosis: A Real-world Case Series
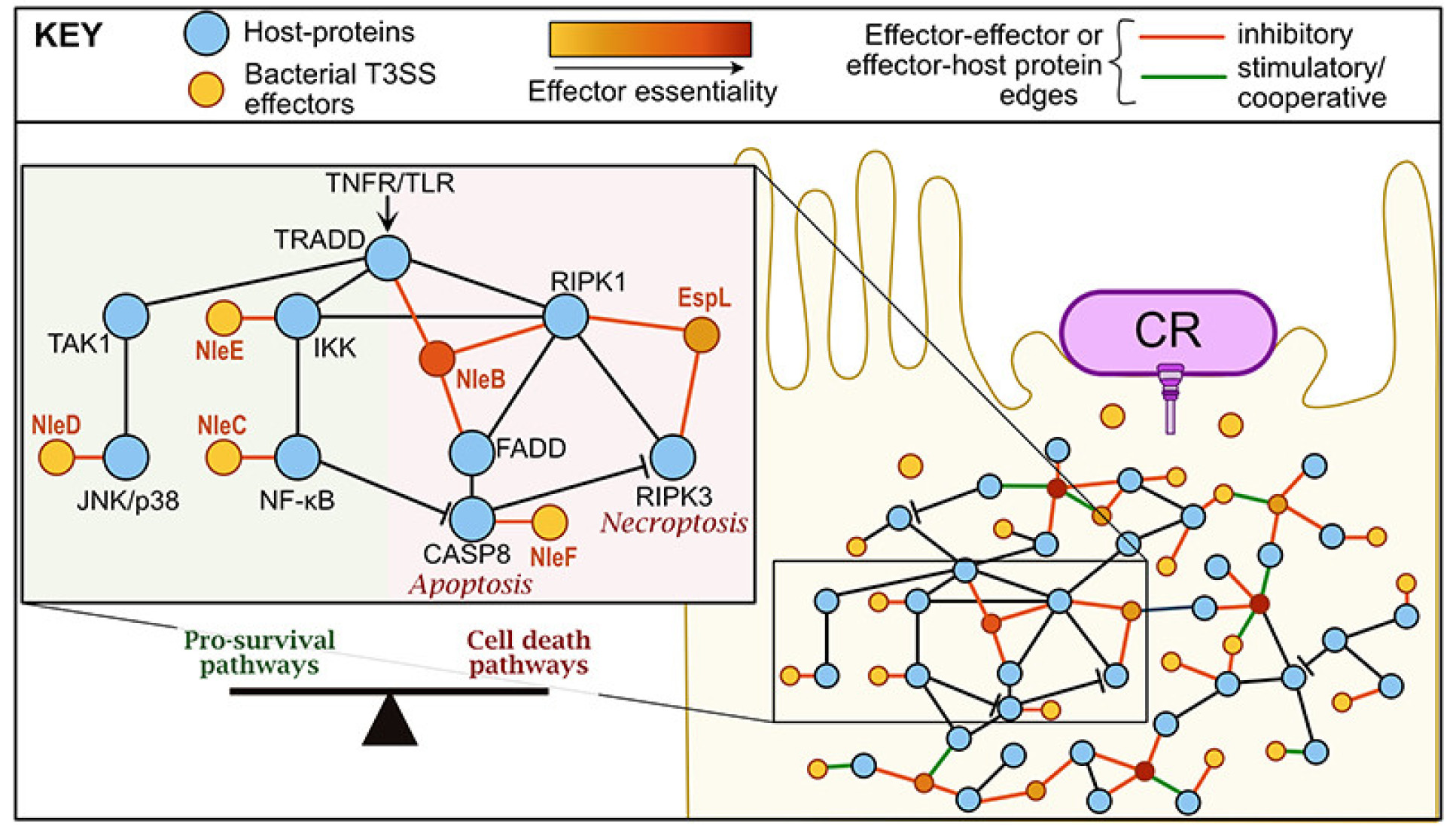
- Type III secretion system effectors form robust and flexible intracellular virulence networks
- Airway macrophage-intrinsic TGF-β1 regulates pulmonary immunity during early-life allergen exposure
- Differential and longitudinal immune gene patterns associated with reprogrammed microenvironment and viral mimicry in response to neoadjuvant radio...
- Single-cell transcriptomics identifies an effectorness gradient shaping the response of CD4+ T cells to cytokines
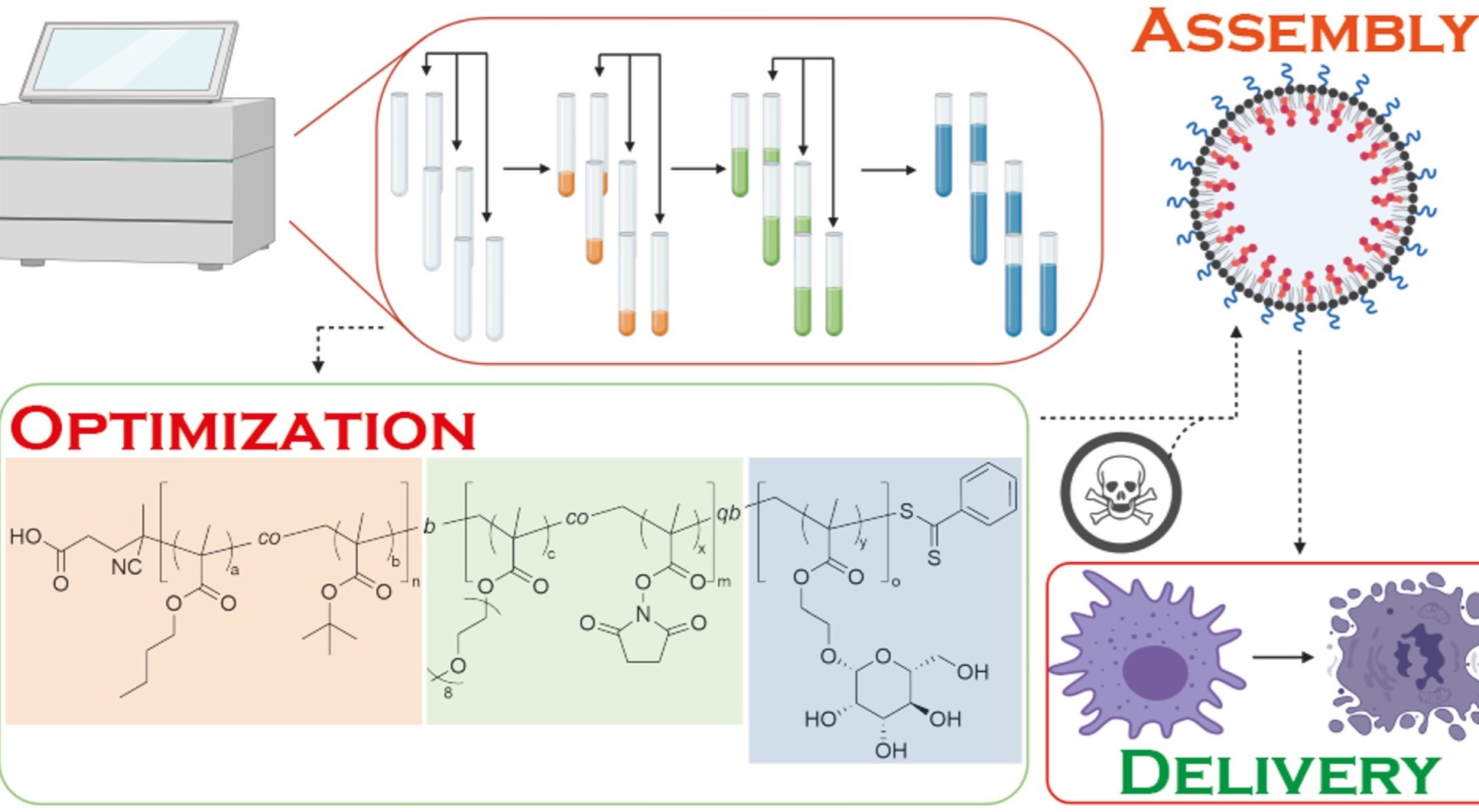
Glycosylated nanoparticles derived from RAFT polymerization for effective drug delivery to macrophages
Rushworth, J.; Montgomery, K.; Cao, B.; Brown, R.; Dibb, N.; Nilsson, S. K.; Chiefari, J.; Fuchter, M. J. ACS Appl. Bio Mater. 2020, 3, 5775.
Tumour-associated macrophages (TAMs) have recently re-emerged as a viable target in cancer therapy. An abundance of this cell type is correlated with poor prognosis in a range of cancers, including breast, prostate, ovarian and non-small cell lung cancers. In this work we utilised reversible addition-fragmentation chain transfer (RAFT) polymerisation and high-throughput methodology to access a range of cross-linked amphiphilic drug delivery systems that were able to self-assemble into micelles and higher-order structures such as polymersomes, whereby novel and literature-known drugs could be loaded onto the hydrophobic core. We demonstrated that the prepared polymers could be assembled into nanoparticles and were successfully internalized into macrophages, in part, via the macrophage mannose receptor (CD206). Finally, we showcased the developed nanoparticles in the delivery of an FMS inhibitor to cells, resulting in inhibition of the FMS receptor. As such, this study laid the groundwork for further drug delivery studies aimed at specifically targeting TAMs with molecularly targeted therapeutics.
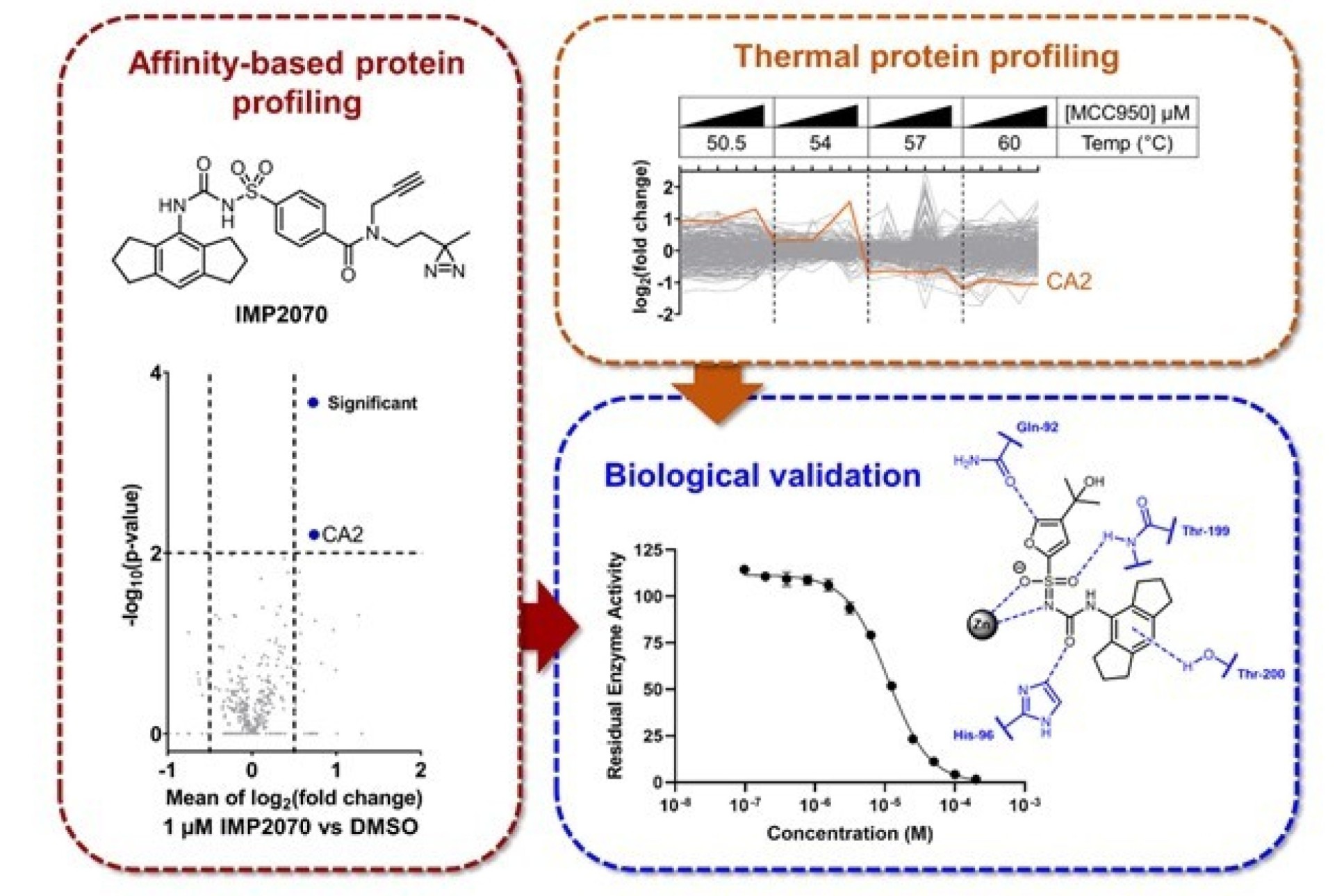
A Probe for NLRP3 Inflammasome Inhibitor MCC950 Identifies Carbonic Anhydrase 2 as a Novel Target
Cassandra R. Kennedy, Andrea Goya Grocin, Tristan Kovačič, Ravi Singh, Jennifer A. Ward, Avinash R. Shenoy, and Edward W. Tate. ACS Chem. Biol. 2021, 16, 6, 982–990
The inflammasome is a multiprotein signalling complex that is activated in response to infection, cellular damage, or stress and is implicated in a range of autoimmune and inflammatory disorders. Current clinical inhibitors of inflammasome pathways are not specific to particular stimuli or to cell death driven by a specific class of inflammasome. As a result, there has been a lot of interest in the development of specific small molecule inflammasome inhibitors, particularly those of the NLRP3 (nucleotide binding and oligomerization domain, leucine-rich repeat and pyrin containing protein 3) inflammasome. One such potent, small molecule inhibitor is MCC950 which has shown encouraging results in a variety of animal models including for multiple sclerosis, Alzheimer’s and Parkinson’s diseases and no off-target effects had previously been identified.
This paper describes the first proteome-wide unbiased target profiles for MCC950. Dr Cassandra Kennedy designed and synthesised a novel photoaffinitybased probe (AfBP) for MCC950 (IMP2070) and showed that it directly engages with NLRP3 and retains inhibitory activity against the NLRP3 inflammasome. De novo target identification with IMP2070 and thermal proteome profiling further identified multiple potential MCC950 off-targets including carbonic anhydrase 2 (CA2), and biochemical assays confirmed MCC950 as a non-competitive CA2 inhibitor.
This work highlights potential biological mechanisms through which MCC950, and derivatives, may exhibit off-target effects in preclinical or clinical studies and will be useful in the interpretation of both prior and future in vitro and in vivo studies using MCC950.
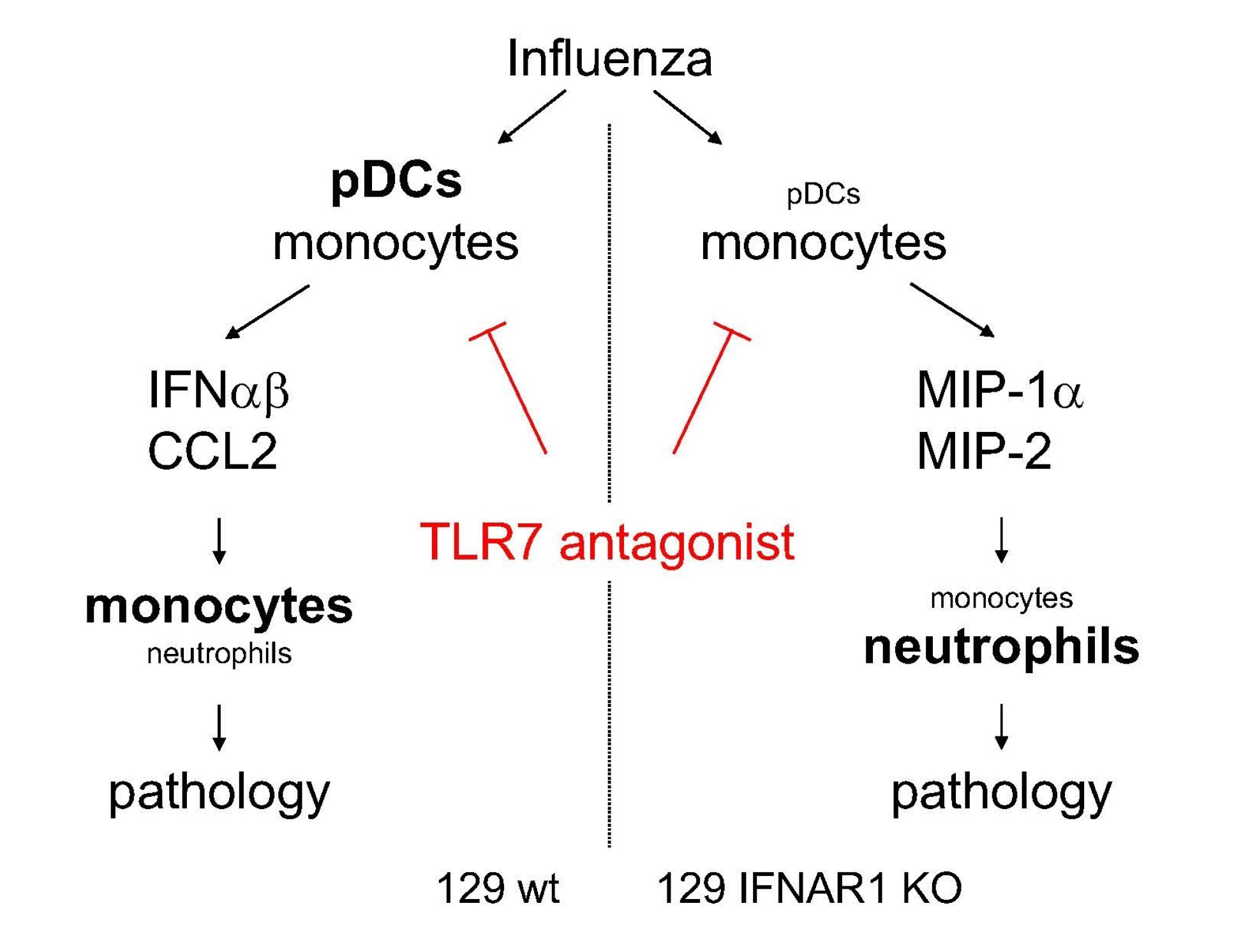
A TLR7 antagonist restricts interferon-dependent and -independent immunopathology in a mouse model of severe influenza
Rappe JCF, Finsterbusch K, Crotta S, Mack M, Priestnall SL, Wack A. J Exp Med. 2021 Nov 1;218(11):e20201631. doi: 10.1084/jem.20201631. Epub 2021 Sep 2.PMID: 34473195
An overly aggressive inflammatory response is associated with increased mortality in diseases such as severe flu and COVID-19. Since influenza virus is sensed by TLR7 in some cells including monocytes and plasmacytoid DCs, and by Rig-I in other immune cells and infected epithelia, the Wack lab used models of severe influenza infection to test whether specific TLR7 antagonism may be beneficial. They showed that blocking the TLR7 pathway, without affecting RIG-I-dependent antiviral responses in lung epithelia, reduced inflammation and mortality in severe influenza without compromising virus control. While the initial assumption was that the TLR7 antagonist would reduce excessive interferon production that can drive immunopathology, the authors found in IFNAR1-deficient mice that TLR7 antagonism also alleviated interferon-independent inflammation. This indicates that immune-mediated pathology in different influenza-infected hosts can have different drivers and different characteristics and that TLR7 antagonism is beneficial against several types of immunopathology in severe influenza. As TLR antagonists are in clinical trials, these results may indicate treatment options for severe influenza. It is an open question whether SARS-CoV-2 is detected in different cell types by different sensing systems, which would allow pursuing a similar strategy.
A mathematical model to identify optimal combinations of drug targets for dupilumab poor responders in atopic dermatitis
Miyano, T., Irvine, A.D. and Tanaka, R.J. (2021) Allergy.
We developed a mathematical model to conduct model-based meta analysis of recent clinical trials for nine biological drugs of atopic dermatitis (AD, a chronic skin disease).
The model describes AD pathogenesis and reflects variations in pathophysiological backgrounds of AD patients. Computer simulation of the model allows us to investigate the mechanisms behind patient variability in drug response.
Our model serves as a computational platform for model-informed drug development for precision medicine, patient stratification, identification of predictive biomarkers and a systems-level understanding of drug effects.
Prolonged residence of an albumin–IL-4 fusion protein in secondary lymphoid organs ameliorates experimental autoimmune encephalomyelitis
Ishihara, A., Ishihara, J., Watkins, E.A. et al. Nat Biomed Eng 5, 387–398 (2021)
Multiple sclerosis (MS) is an autoimmune disease of the central nervous system, which affects millions worldwide, can cause debilitating symptoms for patients. In patients with MS, autoreactive immune cells infiltrate the central nervous system and cause damage. Recent studies have shown that Th17 cells, immune cells that are activated in the body's secondary lymphoid organs, migrate to the brain and play a role in the severity of the disease. Interleukin-4 (IL-4), an anti-inflammatory cytokine, is known to suppress Th17. To use it as a potential therapy, we found a way to keep the IL-4 in the secondary lymphoid organs to ensure that Th17 cells were suppressed and did not migrate. To do this, we recombinantly fused IL-4 to a serum albumin and injected it into mice that had the mouse model of MS and found that it caused the IL-4 to stay within the secondary lymphoid organs. The result was reduced infiltration of Th17 cells into the spinal cord. That suppressed the disease and resulted in fewer symptoms. This is the first time to show how the fusion of this protein to immunosuppressive cytokines can treat and prevent multiple sclerosis.
Application of direct stochastic optical reconstruction microscopy (dSTORM) to the histological analysis of human glomerular disease
Garcia, E., Lightley, J., Kumar, S., Kalita, R., Gőrlitz, F., Alexandrov, Y., Cook, T., Dunsby, C., Neil, M.A., Roufosse, C.A. and French, P.M. (2021). J Pathol Clin Res, 7: 438-445.
The Photonics Group in the Physics Department at Imperial is developing robust, cost-effective, modular open-source microscopy to widen access to advanced light microscopy techniques and to make such instrumentation more sustainable. Within this openScopes.com platform, we are developing low-cost single molecule localisation microscopy to provide super-resolved imaging, including immuno-labelled tissue sections using a technique we describe as easySTORM. In a collaboration with the Department of Inflammation and Immunology, we have been exploring the potential of super-resolved immunofluorescence imaging of histological sections (described as “histoSTORM”) to replace or complement electron microscopy (EM) in the diagnosis of disease, particularly kidney disease.
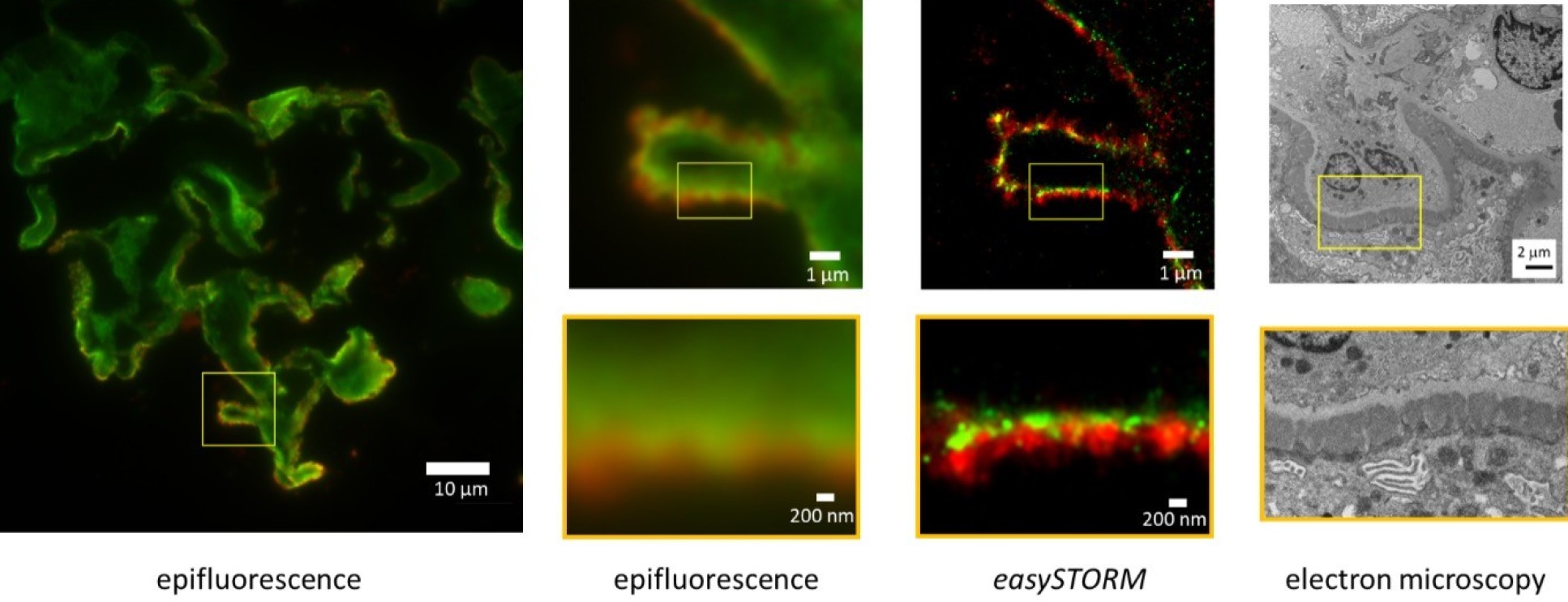
In the UK EM is undertaken following immunofluorescence imaging as a vital tool for the diagnosis of human glomerular diseases but this use of EM is limited to specialised laboratories and it is not available in many other countries. Since easySTORM can provide resolution below 50 nm in biological specimens, including clinical histological sections stained with standard immunofluorescence techniques, we reasoned that histoSTORM may provide an alternative means to resolve tissue ultrastructure to aid the diagnosis of kidney disease where EM is not available. We have applied histoSTORM to FFPE and frozen human kidney biopsy sections stained with clinically approved immunofluorescent probes for the basal laminae and immunoglobulin G deposits. The figure above (adapted from our J. Pathology: Clinical Research paper) shows standard wide-field epifluorescence images of immunolabelled membranous glomerulonephritis together with histoSTORM and EM images, demonstrating the potential of histoSTORM to provide additional information compared to conventional immunofluorescence microscopy. We believe that conventional widefield immunofluorescence microscopes can be upgraded to provide super resolved image information to aid the diagnosis of human glomerular disease with only minor modifications in established immunofluorescence protocols of clinical frozen renal biopsies. histoSTORM can also be applied to image the SARS-CoV-2 virus in histological samples and may add value to the study and/or diagnosis of a wide range of diseases.
Autologous Hematopoietic Stem Cell Transplantation in Active Multiple Sclerosis: A Real-world Case Series
Nicholas RS, Rhone EE, Mariottini A, Silber E, Malik O, Singh-Curry V, Turner B, Scalfari A, Ciccarelli O, Sormani MP, Olavarria E, Mehra V, Gabriel I, Kazmi MA, Muraro P. Neurology. 2021 Aug 31;97(9):e890-e901. doi: 10.1212/WNL.0000000000012449. Epub 2021 Jul 12. PMID: 34253634; PMCID: PMC8408506
Multiple sclerosis (MS) is an inflammatory (presumed autoimmune) disorder of the brain and spinal cord. Recent trials demonstrate that high-dose immunosuppression followed by autologous haematopoietic stem cell transplantation (AHSCT) markedly reduces the disease attacks and lesion development; and improves disability in people with relapsing-remitting forms of MS. Studies on the reconstitution of adaptive immune repertoires support the notion of immune regeneration after autologous haematopoietic stem cell transplantation and have identified mechanisms potentially underlying the treatment effect: depletion of pro-inflammatory T cells and regeneration of naïve T cells in the peripheral blood and cerebrospinal fluid. To begin exploring the question if AHSCT could be an effective and safe alternative to contemporary biological therapies approved for RRMS, or could benefit a subgroup of patients with active progressive MS, we examined outcomes in patients with severe, treatment-refractory MS who we followed in an observational cohort of 120 subjects. At four years after treatment, 87% were free from MS relapses and 85% had no new brain lesions; the neurological function hand not worsened in 65%. There were three treatment-related deaths (2.5%). In this real-world cohort, outcomes after AHSCT are similar to those reported clinical trial populations, although the risks may be higher.
Type III secretion system effectors form robust and flexible intracellular virulence networks
Ruano-Gallego D, Sanchez-Garrido J, Kozik Z, Núñez-Berrueco E, Cepeda-Molero M, Mullineaux-Sanders C, Naemi-Baghshomali Clark J, Slater SL, Wagner N, Glegola-Madejska I, Roumeliotis TI, Pupko T, Fernández LÁ, Rodríguez-Patón A, Choudhary JS, Frankel G. Science. 2021 Mar 12;371(6534):eabc9531. doi: 10.1126/science.abc9531. PMID: 33707240.
Type III secretion system effectors underpin the infection strategy of many clinically important Gram-negative pathogens. For the last 20 years, the main approach to understanding effector-mediated pathogenesis has been to piece together their individual functions and unveil infection mechanisms. However, in contrast to this strategy, this work has shown, using the enteric mouse pathogen Citrobacter rodentium (CR) encoding 31 effectors, that they form a robust and flexible intracellular signalling network in intestinal epithelial cells, which inhibits cytokine secretion from immune cells in the gut.
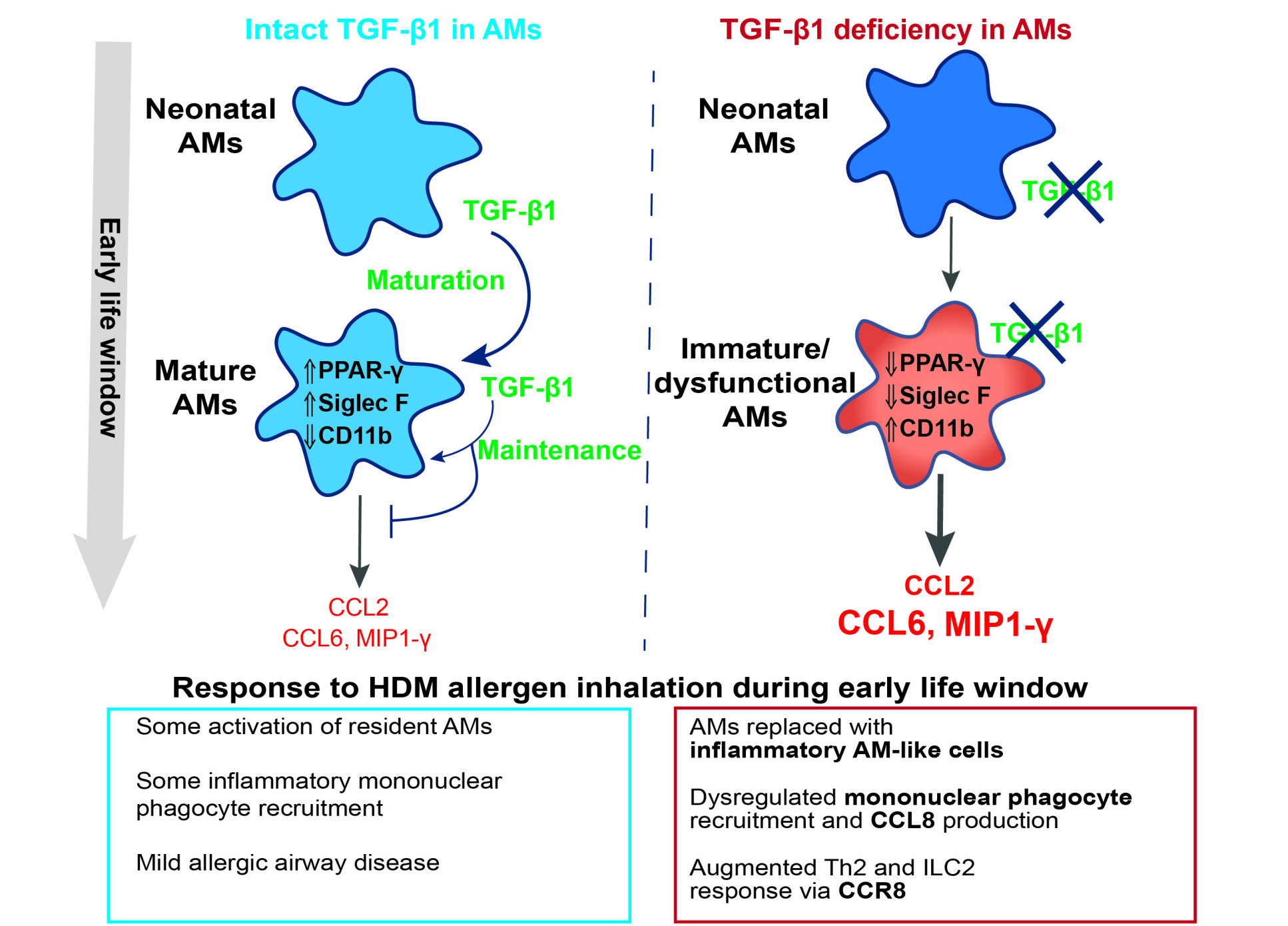
Airway macrophage-intrinsic TGF-β1 regulates pulmonary immunity during early-life allergen exposure
Branchett WJ, Cook J, Oliver RA, Bruno N, Walker SA, Stölting H, Mack M, O'Garra A, Saglani S, Lloyd CM. Airway macrophage-intrinsic TGF-β1 regulates pulmonary immunity during early-life allergen exposure. J Allergy Clin Immunol. 2021 May;147(5):1892-1906. doi: 10.1016/j.jaci.2021.01.026. Epub 2021 Feb 9. PMID: 33571538; PMCID: PMC8098862.
Immune responses in the respiratory tract must be tightly regulated to avoid excessive inflammation that can cause host tissue damage, without compromising immune protection against airborne pathogens. This balance is particularly crucial in early life, a period when many inhaled allergens and pathogens are encountered for the first time.
Airway macrophages (AMs) are specialised tissue-resident immune cells that develop postnatally in the airspaces of the lungs and are key players in regulation of pulmonary immunity. Although the factors controlling the development and balance between pro-inflammatory and immunoregulatory functions of AMs are not completely understood, the cytokine TGF-β1 has previously been implicated in the development of murine AMs. Notably, AMs themselves are major TGF-β1 producers in the lung, supporting a possible autocrine function of this cytokine in regulating AM development and function.
In our study, we used genetically altered mice lacking TGF-β1 gene expression in AMs (Tgfb1∆CD11c mice)to investigate the role of AM-intrinsic TGF-β1 in early life. These mice were able to initially populate the lungs with AMs by day three of life, indicating that intrinsic TGF-β1 was not required for these cells to reach the lung. However, AMs from the knockout mice were unable to adopt and maintain a mature AM phenotype over the first weeks of life – displaying disrupted homeostatic surface marker expression and increased monocyte-attractant chemokine expression. This resulted in an early life window in Tgfb1∆CD11c mice where AMs were present but with a perturbed phenotype. We also observed a transcriptional signature of TGF-β1 responsiveness in paediatric human AMs, suggesting a potential common mechanism of AM regulation in humans and mice.
To investigate the potential consequences of this early life window of AM dysfunction, we repeatedly exposed neonatal Tgfb1∆CD11c and control mice to an extract of the common aeroallergen house dust mite (HDM) via the airways, to model early life allergic inflammation. Consistent with their dysfunction in resident AMs, Tgfb1∆CD11c mice displayed augmented accumulation of inflammatory mononuclear phagocytes in the airway niche following HDM exposure and these mononuclear phagocytes expressed the pro-allergic chemokine CCL8. Notably, while the HDM exposure regimen led to only mild allergic airway disease in littermate control mice, several hallmark features of allergic airway disease were elevated in HDM-exposed Tgfb1∆CD11c mice, including increased Th2 and ILC2 responses and airway wall remodelling. Therapeutic blockade of the CCL8 receptor CCR8 ameliorated these allergic airway disease parameters in Tgfb1∆CD11c mice, in support of a link between heightened CCL8 production and more severe allergic airway responses to HDM in the context of early life AM dysfunction due to TGF-β1 deficiency.
Our results highlight the importance of appropriate AM maturation and function for regulation of immunity to airborne allergens in early life and demonstrate the importance of TGF-β1 as a key factor in this regulation.
By Dr Will Branchett
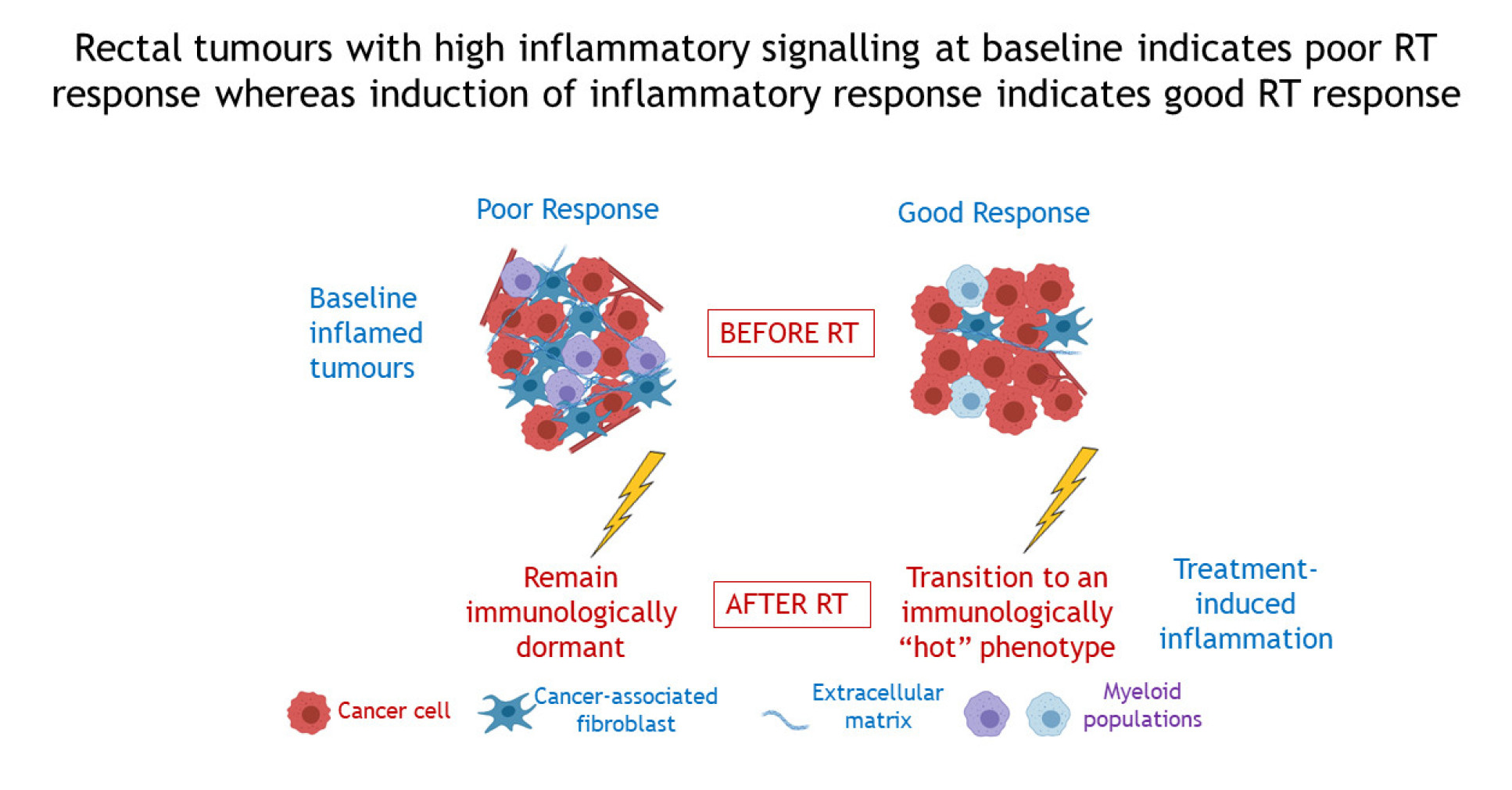
Differential and longitudinal immune gene patterns associated with reprogrammed microenvironment and viral mimicry in response to neoadjuvant radiotherapy in rectal cancer
Rectal cancers show a highly varied response to neoadjuvant radiotherapy/chemoradiation (RT/CRT) and the impact of the tumour immune microenvironment on this response is poorly understood. This study asked how immune gene expression profiling (GEP) may identify differences in expression levels of genes relevant to different radiotherapy responses: (1) at baseline between poor and good responders, and (2) longitudinally from pre-radiotherapy to post-radiotherapy samples. We used a novel unbiased quantitative method to identify tumours with a good versus poor radiotherapy response. Subsequently we showed that genes upregulated at baseline in poor responders represented specific stromal and myeloid populations, as well as pathways of epithelial to mesenchymal transition, coagulation, complement activation and apical junction pathways, which were validated in external cohorts. Longitudinal pathway analysis suggested viral-like pathogen responses occurred in post-treatment resected samples compared with pretreatment biopsies in good responders. This study suggests potentially druggable immune targets in poor responders at baseline and indicates that tumors with a good RT/CRT response reprogrammed from immune "cold" towards an immunologically "hot" phenotype on treatment with radiotherapy.
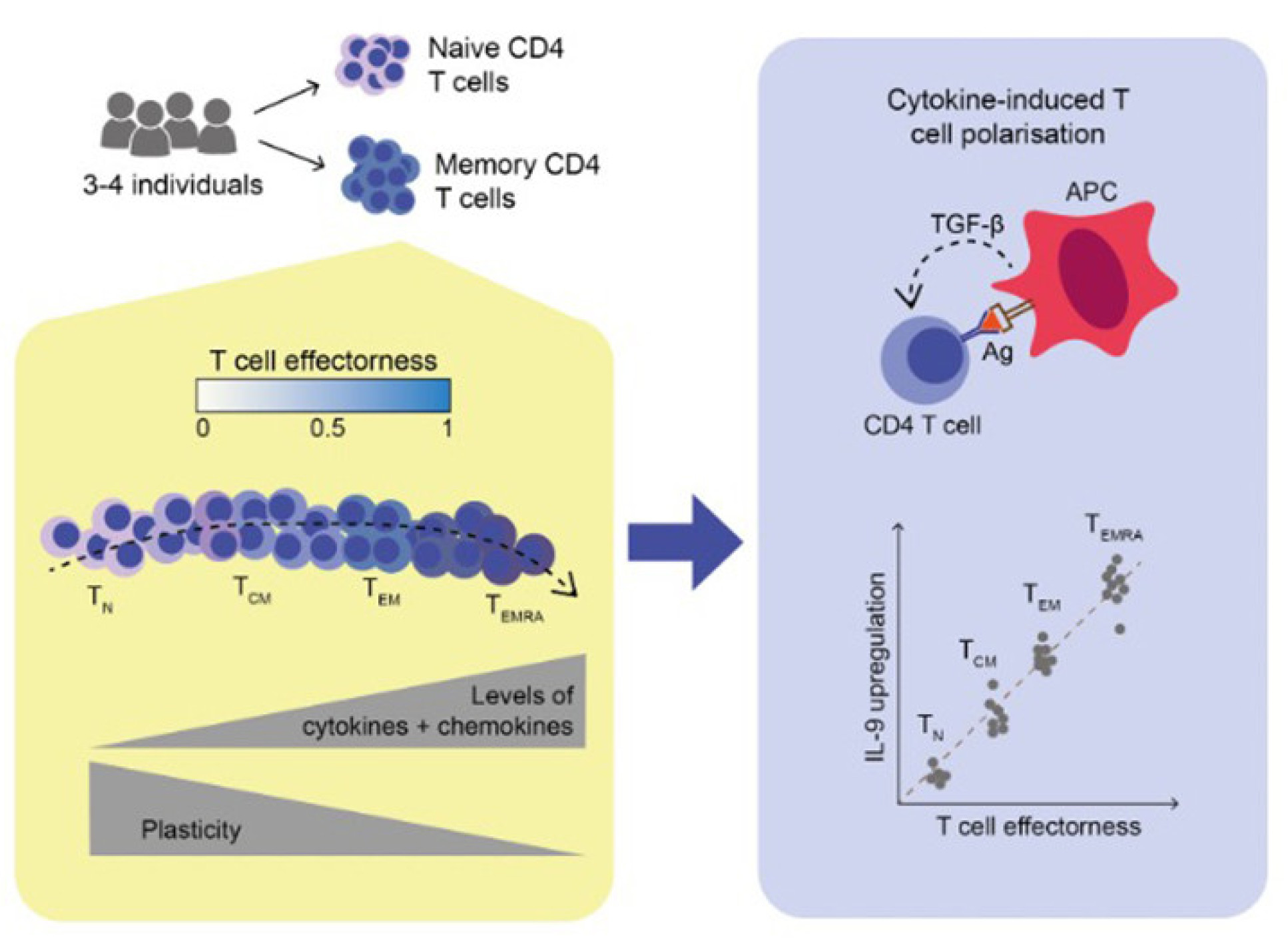
Single-cell transcriptomics identifies an effectorness gradient shaping the response of CD4+ T cells to cytokines
Cano-Gamez, E., Soskic, B., Roumeliotis, T.I. et al. Nat Commun 11, 1801 (2020)
CD4+ T cells play a key role in coordinating the immune response to infection, and their dysfunction can lead to immune-mediated diseases like autoimmunity. CD4+ T cells perform their function by cross-talking with other immune cells, ultimately altering their phenotypes and coordinating their recruitment to the site of infection. This crosstalk is mediated by cytokines and relies on the high degree of plasticity which CD4+ T cells exhibit. Indeed, CD4+ T cells are amongst the most adaptable cells in the human body and are known to acquire different phenotypes in response to different immunological insults. A plethora of mouse and human studies have focused on dissecting this process, and have shown that upon stimulation naive CD4+ T cells adopt different memory phenotypes which match the cytokines present in their microenvironment. For example, stimulation in the presence of IL-12 results in memory T cells that secrete IFN-γ (Th1 cells), while stimulation in the presence of IL-4 results in memory cells that secrete IL-4 (Th2 cells). This process, referred to as T cell polarization, underlies the differential responses observed in viral versus bacterial infections, as well as in allergies or autoinflammation, and has resulted in the advent of the T helper differentiation paradigm. This paradigm has guided T cell research over the last four decades.
While this framework has proven useful, multiple studies have shown that the phenotype of memory T cells is not stable, but plastic. For instance, Th17 cells can be reprogrammed to a Th1-like phenotype by restimulation in a different cytokine environment, and similarly, regulatory T cells can lose their original phenotype if exposed to pro-inflammatory cytokines. The extent of memory T cell plasticity is not yet understood, although it is assumed to be lower than that of naive cells. In this study, we attempted to characterize the plasticity of memory CD4+ T cells by systematically mapping their response to a variety of cytokine environments and comparing it to the response of naive CD4+ T cells. This was done by profiling the full range of RNA and protein changes observed in response to cytokines in these cell populations at two points in time. We observed that memory T cells are indeed plastic. In particular, we showed that stimulation of memory T cells in the presence of TGF-β, known to induce an anti-inflammatory phenotype in naive cells, results in a pro-inflammatory transcriptional program similar to Th17 cells. However, the plasticity of memory cells is reduced compared to their naive counterparts. This is clearly shown by the fact that memory T cells show no detectable response to IL-4, known to induce a Th2 phenotype in naive cells. These changes were true despite similar levels of expression of the receptors for these cytokines in naive and memory cells, suggesting a more profound difference between both subsets.
We hypothesized that these observations might be driven by fundamental differences in the composition of naive and memory T cell populations. Thus, we profiled the response of these cells at the single-cell level (scRNA-seq). This revealed that memory cells are more heterogeneous than naive cells (TN), comprising at least three subgroups: central memory (TCM), effector memory (TEM), and cytotoxic-like TEMRA cells. Surprisingly, naive and memory cells were transcriptionally very similar, and indeed were connected to each other, forming a progression or continuum. We used computational methods to reconstruct this progression and confirmed that it recapitulates the order in which memory cells are believed to arise (TN → TCM → TEM → TEMRA), and is accompanied by evidence of clonal expansion. This suggests that as T cells are repeatedly exposed to antigen, they gradually change their phenotype, thus advancing in this progression. When mapping the gene expression changes that underlie this phenomenon, we found that as cells advance in this progression they express higher levels of molecules directly involved in effector function, such as cytokines and chemokines. We thus used the levels of these genes to define a quantitative variable called “effectorness”, which reflects the position of each individual T cell along this progression.
Describing T cells based on their effectorness helped us understand their ability to respond to cytokines. In particular, we observed a linear relationship between T cell effectorness and the magnitude of gene expression induced by certain cytokines. For example, the level of IL-9 induced in response to TGF-β was linearly dependent on effectorness. This explained why naive cells did not express this cytokine at all, but memory cells did: as cells became more effector, they tended to produce increasingly higher levels of this cytokine when exposed to TGF-β.
In conclusion, our study proposes a new framework for conceptualizing CD4+ T cells as a continuum, where each cell can be given a quantitative “effectorness” value that reflects its stage in the progression from naive to memory. This value can then be used to predict a cell’s degree of plasticity, as well as the type of response it would mount if exposed to a given cytokine.