REC Ref: 12/LO/0768
Active/ in follow-up and not recruiting.
STUDY OBJECTIVE
The primary objective of the study is to verify the ten-year clinical and radiographic performance of the Furlong Evolution® Hip stem when used in patients under normal conditions and, at the same time, evaluate its safety over a period of 10 years by reporting complications, serious adverse patient or device events.
The secondary objective is to evaluate patient function, satisfaction, and quality of life at each follow-up visit. Assessments will be in the form of questionnaires, such as the Oxford Hip, EuroQol 5D and Harris Hip Score.
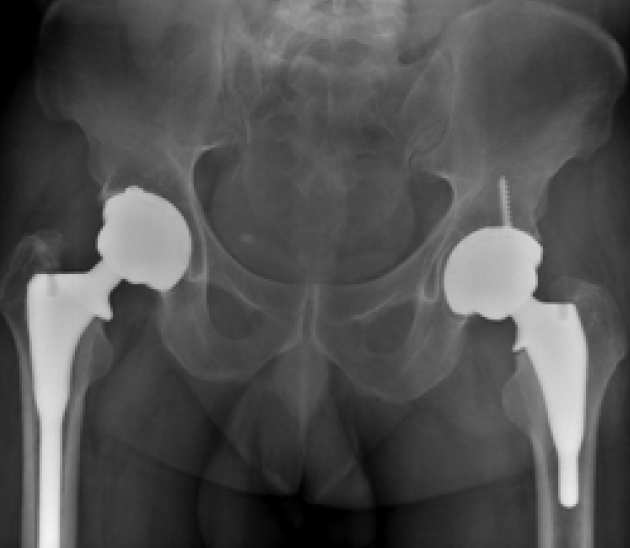
Overview
Total Hip Replacement (THR) is a highly effective procedure for relieving pain and restoring function. The JRI Furlong® HAC femoral stem is one such implant with good long-term survivorship. and published findings have shown a 97.4% survivorship at a mean of 17 years of follow-up in patients.
Our foremost intention is to assess the function and survival of the new Evolution® hip replacement implant. As part of ongoing developments in hip replacement implants, this new modification has been developed from an existing hip replacement stem, which has very good long-term results.
The reason for the development was to try to give the patient a better surgical and rehabilitation experience with a much kinder operation. In theory, this new hip stem might aid post operative recovery and may also perform at least as well as its predecessor. We must stress that the benefits and potential downsides of the Furlong Evolution® Hip Stem are currently unknown and is being evaluated in this study. We wish to test these potential benefits by making a long-term commitment to follow up with patients who consent to the new Furlong Evolution® 10 years after their operation.
Contact
Mr Emmanuel Temilade
Clinical Trial Coordinator
o.temilade@imperial.ac.uk