Tracking malaria parasite growth in mosquitoes may lead to new preventatives

Dr Valerie Chiamaka Ukegbu, Dr Dina Vlachou, and Professor George K. Christophides observe malaria parasites developing within a mosquito
Researchers at Imperial College London have devised a way to concurrently track the function of thousands of malaria parasite genes within mosquitoes.
The team say the technique could uncover fresh insights into potential targets for disrupting parasite development in the insect vector, preventing its ability to transmit malaria to humans. The results are published today in Cell Host and Microbe.
In this proof-of-concept, we studied a small number of genes, but the approach can be scaled up to screen thousands of genes simultaneously. Dr Dina Vlachou
Despite ongoing efforts, malaria remains a critical public health concern. Recent reports indicate a decline in the efficacy of current measures, underscoring the urgency for new interventions.
The parasite that causes malaria goes through various life stages both inside mosquitoes and humans. Knowing the genetic and molecular processes behind parasite development in the mosquito could reveal targets for interventions that would block disease transmission, but picking apart these processes has been difficult.
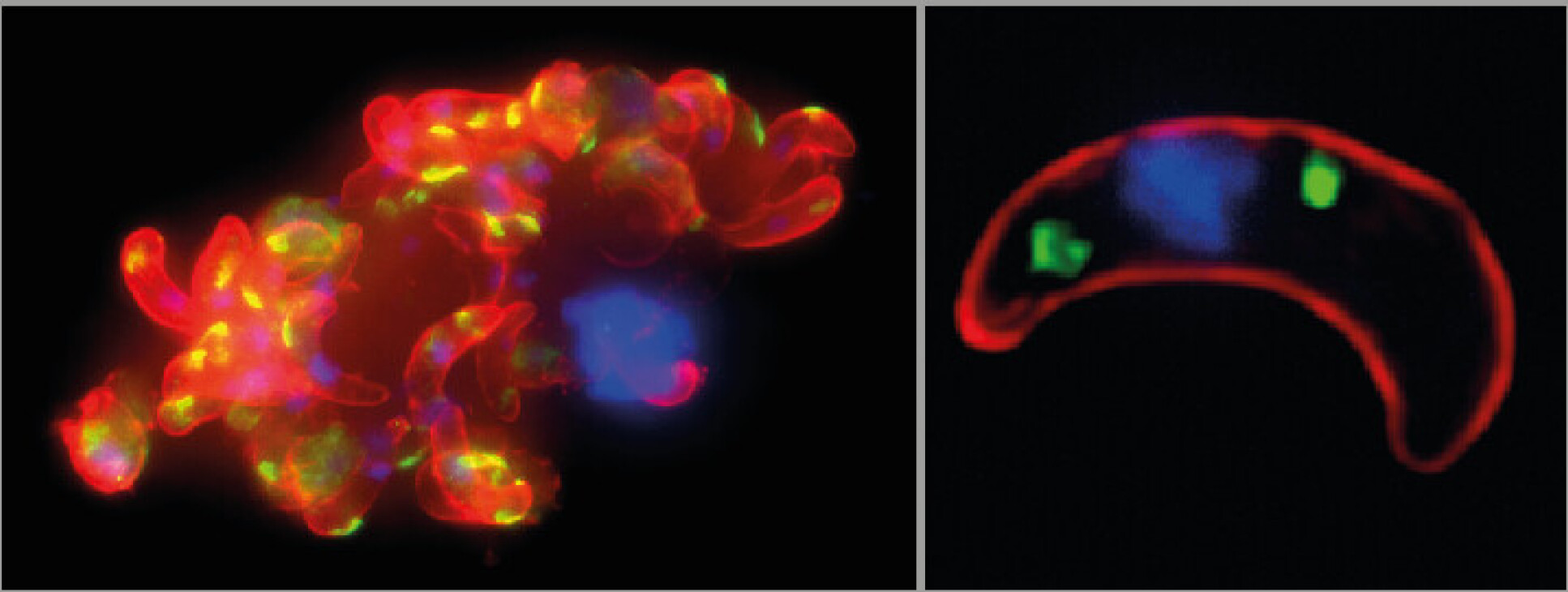
Genetic studies often involve mutating certain genes and observing what effect this has on the developing organism. But inside the mosquito, two opposite-sex gametes (sex cells) of the parasite combine in sexual reproduction, which can introduce a working copy of the mutated gene and ‘rescue’ the resulting mature parasite from detrimental effects.
To overcome this obstacle, the research team led by Dr Dina Vlachou and Professor George K. Christophides from the Department of Life Sciences at Imperial capitalised on their prior discovery that male genes largely remain inactive after reproduction.
Dr Vlachou explained: “We generated batches of exclusively female mutated gametes, which we then crossed with normal male gametes, ensuring the persistence of introduced mutations in resulting mature parasites. In this proof-of-concept, we studied a small number of genes, but the approach can be scaled up to screen thousands of genes simultaneously.”

Crucial genes
As well as confirming the role of previously characterised genes, the team identified new genes that are crucial for malaria transmission.
Postdoctoral researcher Dr Chiamaka Valerie Ukegbu, first author of the study, said: “Among these new genes, mutations in two impede parasite motility, preventing mosquito infection, while mutations in another two obstruct the formation of parasites capable of infecting humans. The latter pair of genes encode proteins situated in the crystalloid, an enigmatic organelle only present in mosquito-stage parasites.”
Professor Christophides added: “Our results confirm that the crystalloid is key to parasite development in the mosquito, pointing to it as an excellent target for impeding disease transmission from mosquitoes to humans.”
The researchers envision this technology as a catalyst of new discoveries, substantially enhancing insights into host-parasite interactions and malaria transmission.
The work was funded by the Wellcome Trust and the Medical Research Council.
-
'Identification of genes required for Plasmodium gametocyte-to-sporozoite development in the mosquito vector’ by Chiamaka Valerie Ukegbu et al. is published in Cell Host and Microbe.
Article supporters
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Hayley Dunning
Communications Division