System overview
We have developed a flexible open source configuration of OPT that can be applied to a wide range of preclinical and clinical imaging applications, including fixed, cleared tissue samples and live organisms such as c elegans, zebrafish. The sample is mounted in a transparent tube that is index-matched to its immersion fluid and this tube is suspended via a magnetic coupling in a square cuvette of the same immersion fluid. The sample is rotated by a top-mounted stepping motor and the system has two imaging arms. One incorporates a telecentric lens of unity or 0.5x magnification (depending on the specific camera and sample size used) and the other is a 4x magnification microscope arm. This system is mainly used for fluorescence OPT although it can be reconfigured for transmission OPT. Excitation of fluorescence can be provided using LED or using a laser. As for all fluorescence imaging instruments, in each case it is important to select excitation and emission filters to stop any excitation light reaching the detectors (cameras). OPT typically entails image exposure times up to a second (depending on excitation source and sample brightness) and can utilise the same cameras as other fluorescence microscopes. We have mainly worked with cooled CCD or sCMOS cameras and are currently exploring the use of CMOS cameras, including recently available fan-cooled CMOS cameras. Recently we published a paper describing OPTImAL: OPT Implemented to for Accessibility and Low-cost, which included our latest list of components we use to build these instruments and links to our OPT instrument control and data reconstruction software. As well as conventional OPT, OPTImAL enables compressive sensing OPT for faster imaging of live samples and an implementation of focal scanning OPT to provide higher spatial resolution.
Dual mode OPT with LED illumination
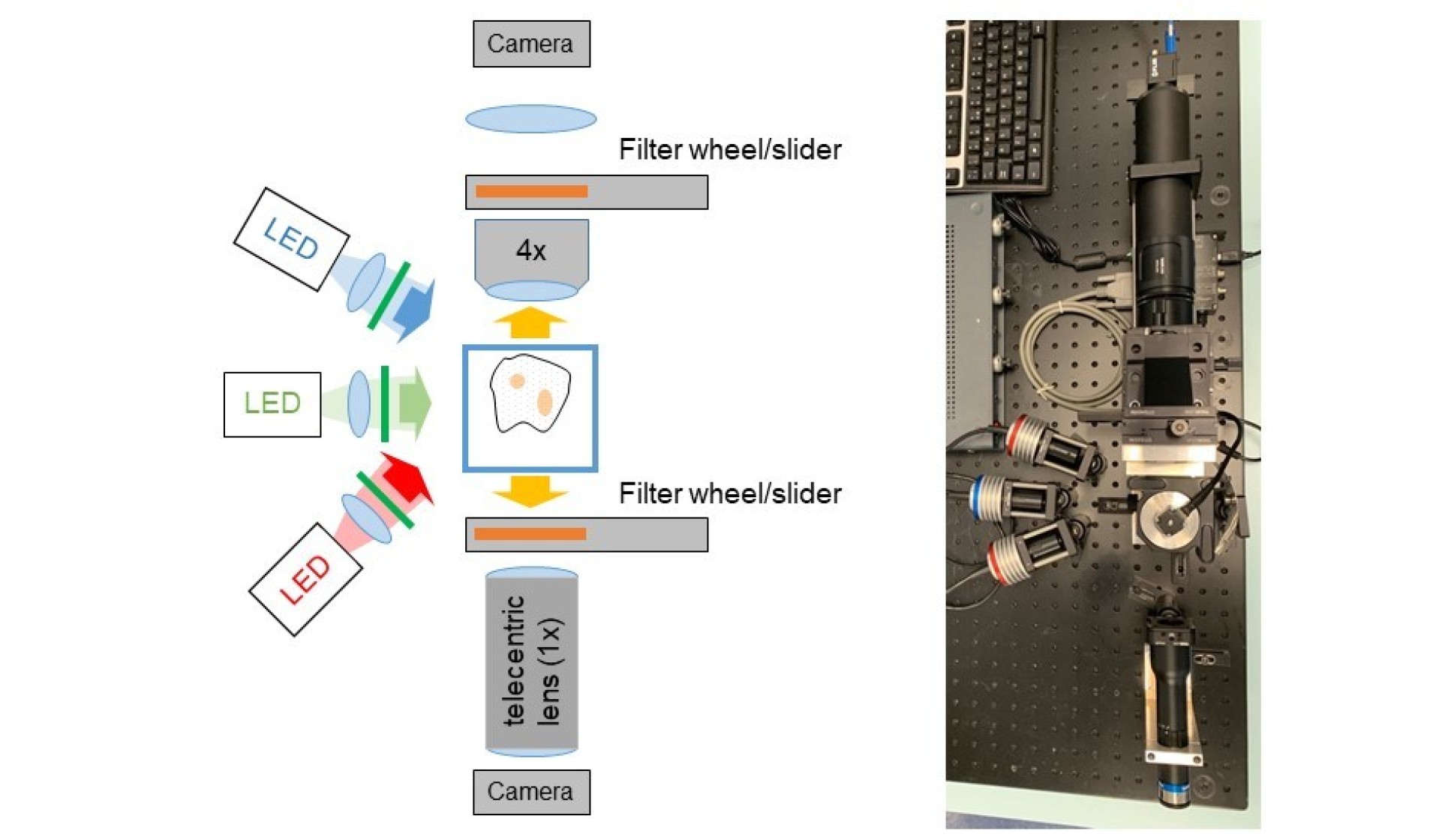 LED illumination is relatively low-cost and low intensity, which reduces photobleaching and phototoxicity. It is convenient for non-specialist laboratories since LED illumination is less potentially hazardous than laser illumination. However, the lower ilumination intensities lead to longer acquisition times, which may be an important consideration when imaging live samples.
LED illumination is relatively low-cost and low intensity, which reduces photobleaching and phototoxicity. It is convenient for non-specialist laboratories since LED illumination is less potentially hazardous than laser illumination. However, the lower ilumination intensities lead to longer acquisition times, which may be an important consideration when imaging live samples.
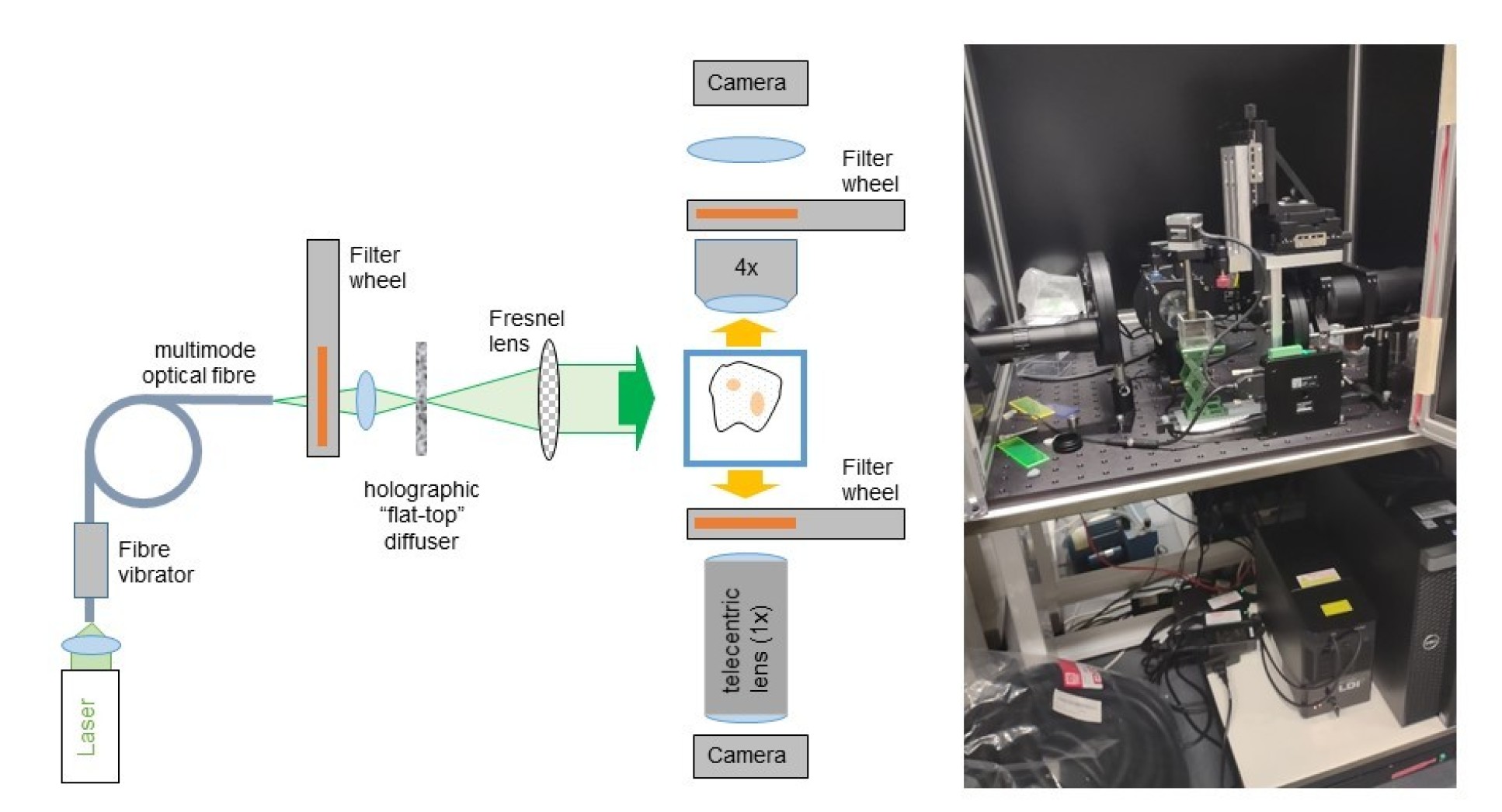
Dual mode OPT with laser illumination
A wide range of lasers can be used for OPT, as with other fluorescence microscope techniques. However, because OPT is a wide-field imaging technique, it is important to illumination the sample with reasonably uniform intensity across the field of view, for which we utilise a "flat-top" holographic diffuser. It is important to average out any laser speckle and this can be done by dynamically varying the speckle pattern on a faster time scale than the image acquisition. We do this by coupling the laser illumination into a multimode optical fibre and vibrating it. There is no requirement for single transverse mode lasers - and multimode lasers are preferable for averaging speckle patterns. We typical utilise a multiline laser source based on multimode diode lasers that are coupled into 600 micron diameter optical fibre. After focussing this laser radiation onto the holographic diffuser, we collimate the laser radiation with a 50 mm diameter Fresnel lens.
OPT software
Our OPT software for OPT data acquisition written in MicroManager will will soon be available here.
OPT data can also be reconstructed using ic_OPTtools.m,. This is part of ALYTools, a collection of open source bioimaging analysis applications written in MATLAB by Yuriy Alexandrov, which is available here.
Alternatively, OPT data can be reconstructed using Radon_Transform.jar, an open source Radon Transform plug-in written by Damien Farrell for ImageJ. Instructions are available for download.