Research | Who we are | Opportunities | Publications | Gallery | Contact
Our Research
(1) We make use of chemical principles to understand fundamentally important reactions in biology. Reduction-oxidation (redox) reactions underpin innumerable chemical reactions and much of the chemistry of life. Many redox reactions proceed via radical intermediates and these are often located in mechanistically key locations (see our recent book chapter). We investigate how oxidation-state changes govern respiration (respiratory complex I) and photosynthesis (photosynthetic complex I) and how nature has fine-tuned the redox properties of its many intricate molecular machines. Membranes play a fundamentally important role for many proteins and we are investigating the role of membranes on protein activity and function through spin labels and protein-intrinsic paramagnetic centres.
(2) We are developing film-electrochemical EPR (FE-EPR) as a new method to study the evolution of radicals during redox and catalytic reactions in real time. This enables detailed and previously impossible mechanistic understanding of both chemical and biochemical reactions. The catalytic reactions we investigate range from well-known long-established chemical transformations such as nitroxide-catalysed alcohol oxidation, to complex metal-containing enzymes that may prove to be novel targets for the development of new antimicrobials. Our FE-EPR methodology is even finding applications in battery research.
(3) Radicals often hold key mechanistic information - but trapping them in sufficient numbers for detailed EPR investigations can be a major challenge. We specialise in working with minute sample concentrations and volumes, and are seeking to make the impossible possible with our current collaborative grant SpinSUPER.
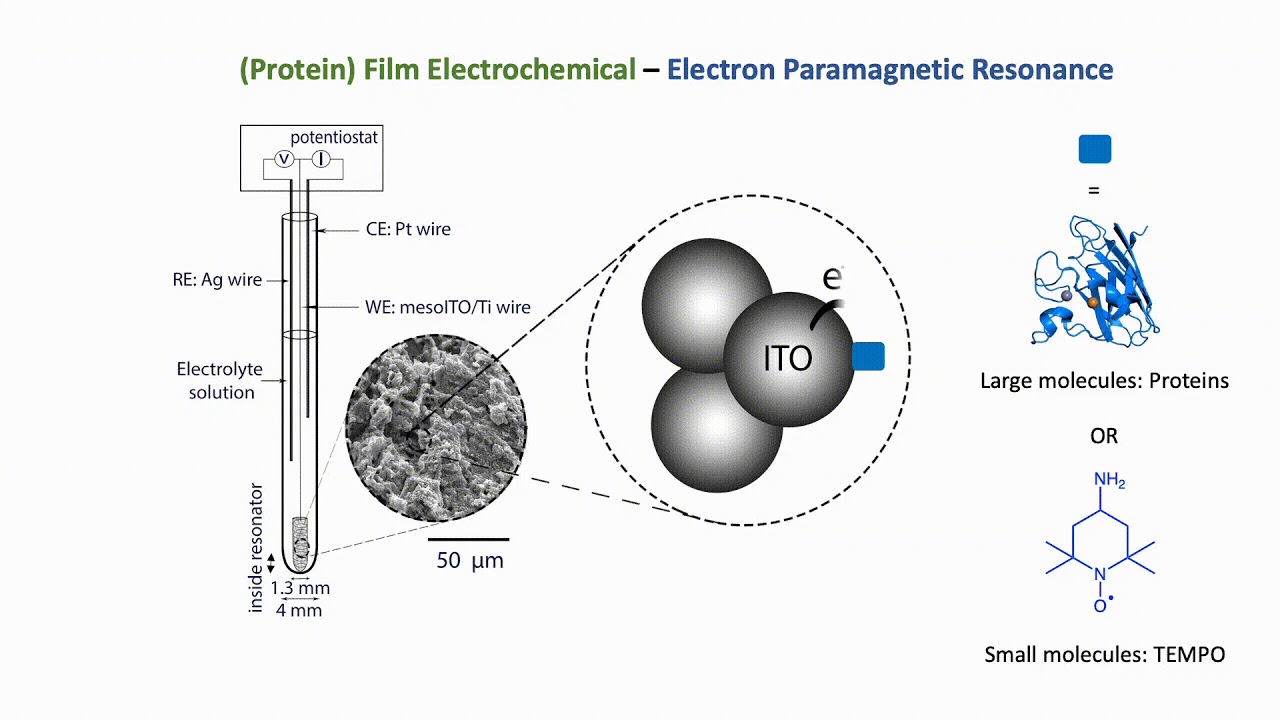
Protein Film Electrochemistry-EPR
PFE-EPR
The combination of electron paramagnetic resonance (EPR) spectroscopy with protein film electrochemistry (PFE) provides a novel platform for the investigation of redox-active species including metalloproteins. The development of PFE-EPR enables the detection of paramagnetic species with direct and accurate potential control, providing new mechanistic insight to redox-based processes in biomolecules, including catalysis. Current EPR spectroelectrochemical methods to study proteins are based on generating radical species in solution and therefore limited by diffusion (precluding accurante potnetial control) and do not enable catalytic investivations. We exploit the advantages of PFE, i.e. 'wiring' electrons directly to the working electrode surface, to eliminate diffusion limitations.
Project members and collaborators
- Adam Sills (PhD student, ICL)
- Yunfei Dang (PhD student, ICL)
- Davide Facchetti (PhD student, ICL)
- Dr. Sam Cobb (PDRA, Cambridge)
- Prof. Erwin Reisner (PI, Cambridge)
Project alumni
- Dr. Maryam Seif Eddine (PDRA, ICL)
- Dr. Kaltum Abdiaziz (PDRA, QMUL)
1. Respiratory Complex I
NADH:ubiquinone oxidoreductase
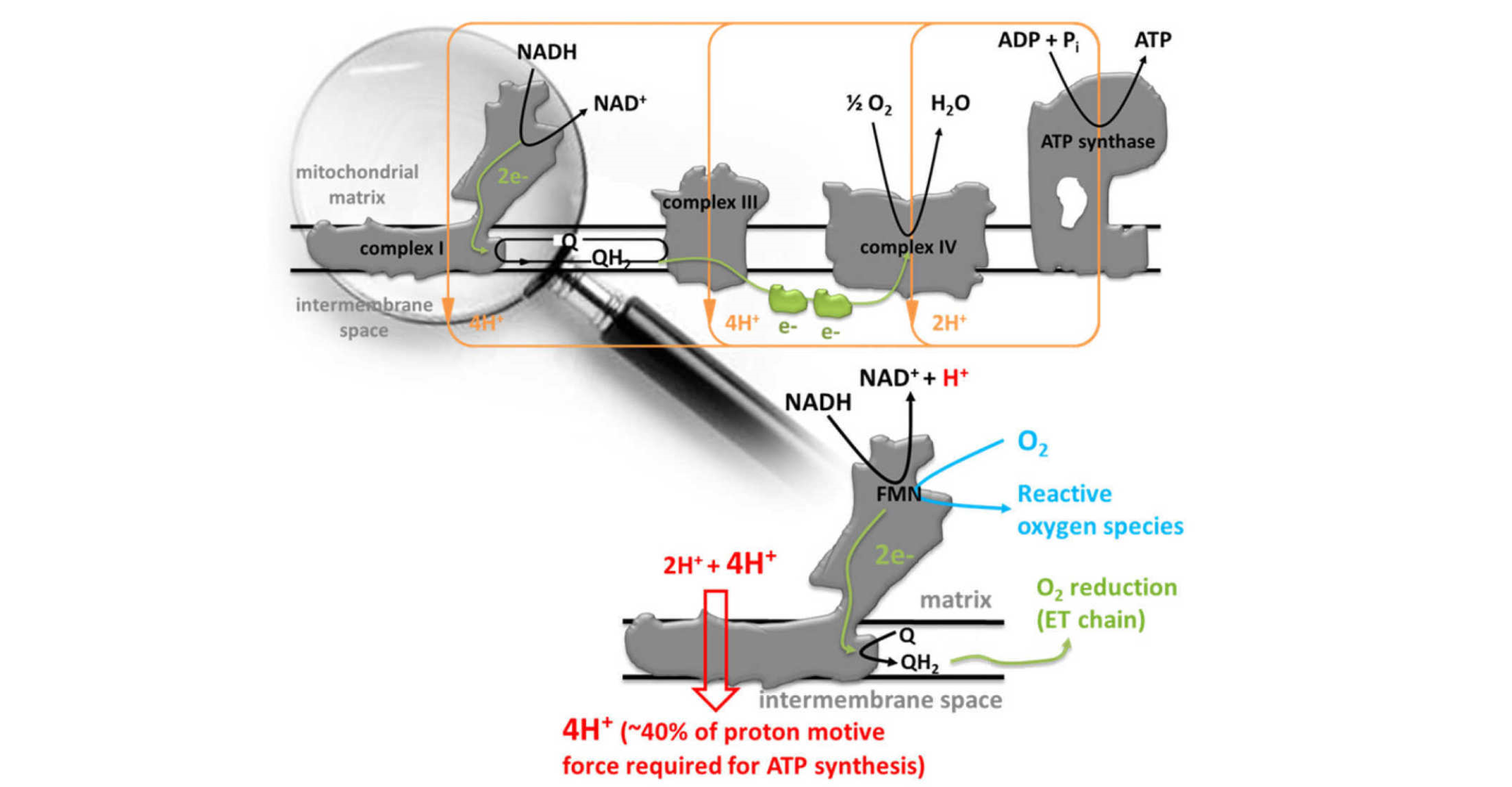 Complex I – the entry point of electrons into the respiratory chain of all higher organisms – is the last of the respiratory enzymes whose mechanism remains unknown. In mitochondria, complex I oxidises NADH from sugars and fats, reduces ubiquinone and transports protons across the inner mitochondrial membrane, thereby contributing to the proton motive force that enables ATP-synthase to make ATP.
Complex I – the entry point of electrons into the respiratory chain of all higher organisms – is the last of the respiratory enzymes whose mechanism remains unknown. In mitochondria, complex I oxidises NADH from sugars and fats, reduces ubiquinone and transports protons across the inner mitochondrial membrane, thereby contributing to the proton motive force that enables ATP-synthase to make ATP.
We are working on elucidating some of the key elements of the mechanism of complex I using advanced EPR methods, in particular we are fascinated how the redox energy built up through electron transfer is used to drive proton translocation. Given the large size of the enzyme (the size of the mitochondrial enzyme is ca. 1 MDa), many biophysical methods routinely applied to study protein cannot be used (e.g. NMR). We are exploiting the fact that EPR spectroscopy is blind to all the many paired electrons in the complex, and that unpaired electrons are situated in mechanistically key locations. For instance, many of the iron-sulfer clusters ([2Fe-2S] and [4Fe-4S] clusters) become EPR-visible when reduced. We have recently divised a potentiometric method that enables us to adjust very small amounts of protein to very precise reduction potentials - an important prerequisite for studying these redox-active proteins spectroscopically. This has enabled us, in conjunction with pulse EPR spectroscopy and site-directly mutagenesis, to investigate the role of Fe-S cluster N2.
Project members and collaborators
- Adam Sills (PhD student, ICL)
- Fang Fang (PhD student, ICL)
- Eleanor Clifford (PhD student, ICL)
- Dr John Wright (PDRA, Medical Research Council, Mitochondrial Biology Unit, Cambridge)
- Dr Judy Hirst (PI, Medical Research Council, Mitochondrial Biology Unit, Cambridge)
Project alumni
- Dr. Maryam Seif Eddine (PDRA, ICL)
- Dr. Kaltum Abdiaziz (PDRA, QMUL)
2. Photosynthetic Complex I
The NAD(P)H dehydrogenase-'like' complex (NDH)
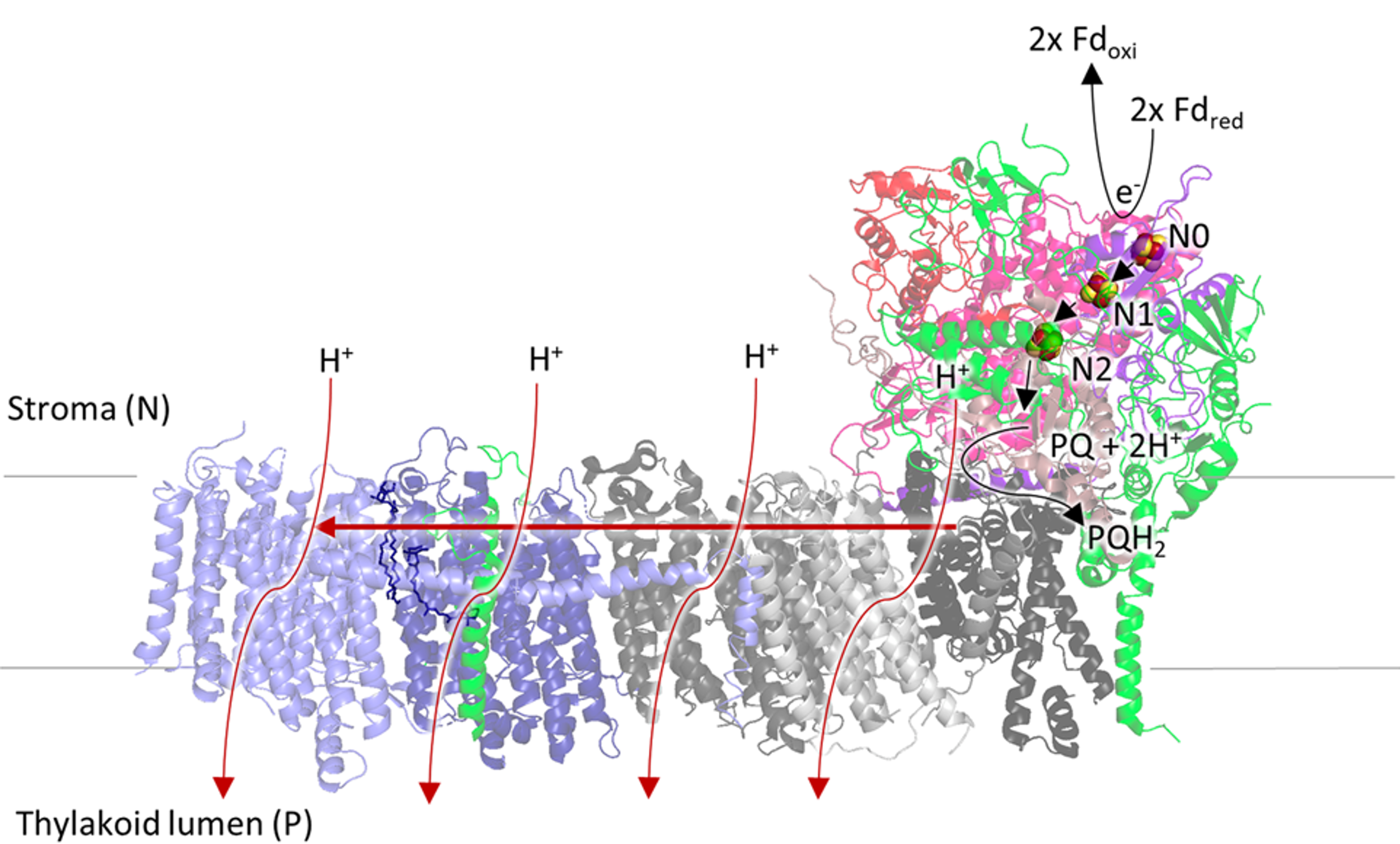
We have teamed up with Dr Guy Hanke to investigate the fascinating molecular machine NDH, in many ways related to the mitochondrial enzyme (complex I, above), present in plants and cyanobacteria. Our goal is to unravel the mechanism of NDH, and ultimately to exploit this knowledge in order to increase stress tolerance in plants (in collaboration with Prof. Nestor Carrillo, Rosario, Argentina). Increasing crop yields is an especially important challenge facing the developing world, but may affect us all one day given the ever rising world population. Research on NDH has thus far focused on genetic investigations and very little is known about the function of NDH, and no biophysical studies are reported. Using our experience with the mitochondrial enzyme, we are using EPR spectroscopy and protein film electrochemistry to investigate the co-factors in and the electron donors to NDH. Moreover, the knowledge gained through NDH is also likely relevant for understanding the mechanism of complex I.
See Dr Hanke's webpage for more details on the biological context.
Project members and collaborators
- Eleanor Clifford (PhD student, ICL)
- Angeliki Chatziathanasiou (PhD student, ICL)
- Dr Guy Hanke (PI, School of Biological and chemical Sciences, QMUL)
Project Alumni
- Gemma McGuire (PhD student, QMUL & ICL)
- Katherina Richardson (PhD student, LIDo program, QMUL & ICL)
Artificial membrane systems: tailor-made tools to study membrane proteins
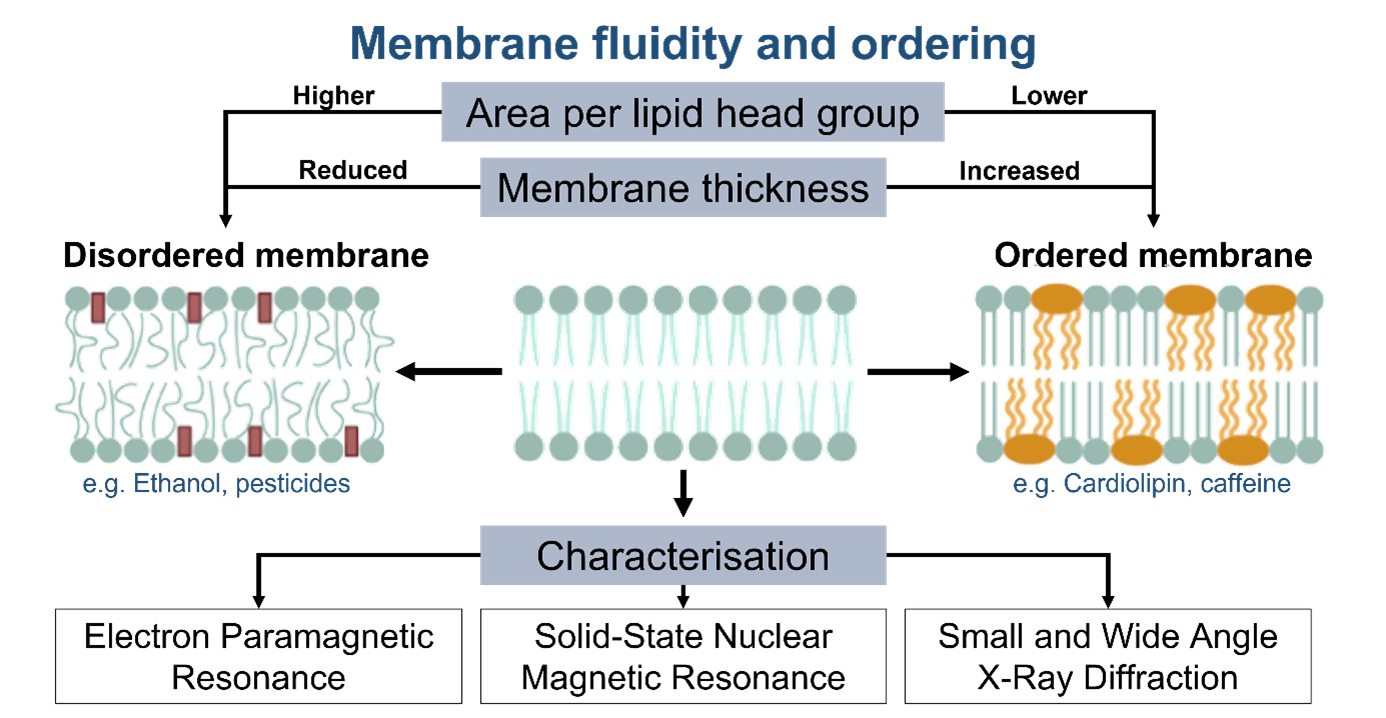
Biological membranes are mainly formed from bilayers of amphiphilic phospholipid molecules and harbour small hydrophobic components, such as vitamins, quinones and pigments. In addition, they can host a huge range of membrane proteins from small, peripherally bound proteins to integral multicomponent megaDalton complexes.[1] The total protein content of a membrane increases with the complexity of the biochemical functions it helps to sustain.
The innate complexity of densely packed biological membranes makes targeted functional studies of individual membrane proteins in their native cellular environment challenging. To overcome this problem, mimetic systems ranging from membrane-monolayers to lipid vesicles (known as liposomes) have been employed.[2] Such systems enable membrane proteins to be studied in relatively simple, well-defined environments while still being orientated and inserted inside a lipid environment needed for full activity.[3]
The complex interaction patterns inside the used lipid bilayer structure participate significantly in the overall performance of the reconstituted protein of interest. Therefore, we (in collaboration with the Membrane Biophysics group) are interested in studying the membrane ordering and fluidity in the pure liposomes to fully unravel the interplay of biomimetic environment.
References
- J. P. Hallett and T. Welton, Chem. Rev., 2011, 111, 3508-3576
- M. Watanabe et.al., Chem. Rev., 2017, 117, 7190-7239
Project members and collaborators
- Jana Eisermann (PDRA, Roessler group)
- John Britten (PhD, Roessler group)
Understanding non-covalent interactions in ionic liquids
 Ionic Liquids (ILs) are a remarkable class of liquids, receiving huge attention for their potential in a range of applications such as synthesis and electrochemistry.[1,2] The most common ILs are composed of an inorganic anion with an organic amphiphilic cation and can segregate into ionic and non-polar domains. This nano-structure leads to different solvent environments for various solutes, with the combination of non-covalent interactions (e.g. coulombic interactions, hydrogen bonding, π-π interactions and dispersion interactions) known to be important.[3-4]
Ionic Liquids (ILs) are a remarkable class of liquids, receiving huge attention for their potential in a range of applications such as synthesis and electrochemistry.[1,2] The most common ILs are composed of an inorganic anion with an organic amphiphilic cation and can segregate into ionic and non-polar domains. This nano-structure leads to different solvent environments for various solutes, with the combination of non-covalent interactions (e.g. coulombic interactions, hydrogen bonding, π-π interactions and dispersion interactions) known to be important.[3-4]
To fully explore the ‘designer solvent’ concept of ILs, we require in-depth understanding of how molecular properties lead to macroscopic behaviours. For dynamic properties of solutes, this remains elusive. It is crucial to find a method to measure both structural and dynamic properties of solutes in ILs, as this could have major impact on their potential applications. Electron paramagnetic resonance (EPR) spectroscopy could be a powerful tool for this goal, as it can directly detect the environment around a solute with unpaired electrons.
EPR spectroscopy offers two main advantages compared to other spectroscopic methods: a) it is incredibly versatile in terms of experimental conditions, since it allows performing measurements over a wide temperature range, without being limited by the physical state of the sample – something that is a common problem with several other spectroscopy techniques (e.g. NMR, UV-Vis or fluorescence spectroscopy); b) ionic liquids are silent substrates for EPR investigations, since they don’t contain any EPR-active groups and, as a result, there are no issues with background noise in the measurements. In this project we (in collaboration with the Tom Welton research group) use nitroxide spin probes as spies to understand the ionic liquids’ nanostructuring and immediate chemical environment around the radical. Using a combination of continuous wave (CW) and pulsed EPR experiments we have investigated a variety of ionic liquid systems, ranging from macroscopically homogeneous ionic liquids to ionic liquid crystals.
References
- J. P. Hallett and T. Welton, Chem. Rev., 2011, 111, 3508-3576
- M. Watanabe et.al., Chem. Rev., 2017, 117, 7190-7239
- R. Hayes et.al., Chem. Rev., 2015, 115, 6357-6426
- M. Y. Ivanov et.al., J. Phys. Chem. Lett., 2018, 9, 4607-4612
Project members and collaborators
- Jana Eisermann (PDRA, Roessler group)
- Spiros Koutsoukos (PDRA, Welton group)
Funding