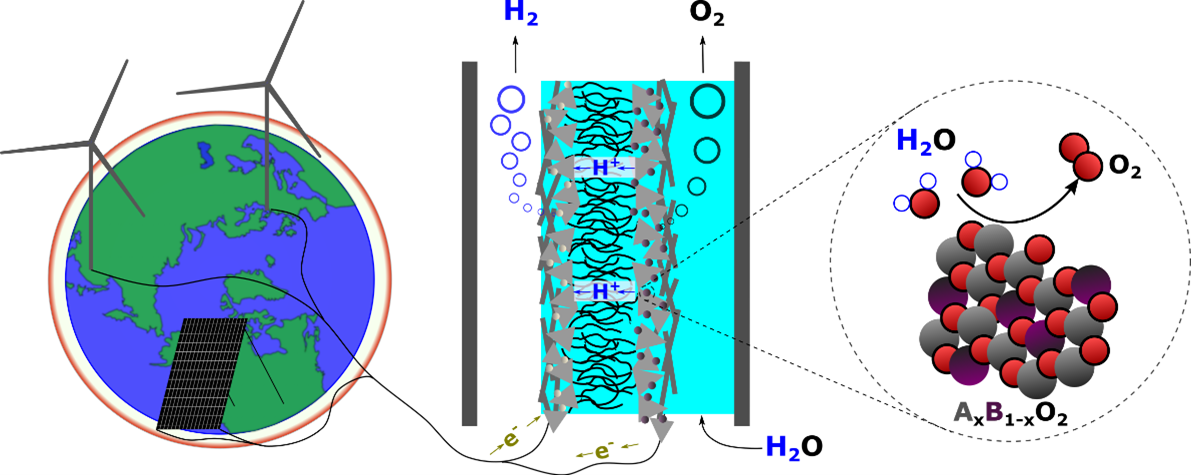
Water electrolysis plays a central role in renewable energy storage as it converts renewable electricity into molecular hydrogen (H2) to be used as a feedstock or fuel. Active, stable, and low-cost electrocatalysts are key to producing hydrogen efficiently and at scale via water electrolysis. Our group focus on understanding the reaction mechanism of water oxidation on metal oxides and developing low-cost and stable OER catalysts based on the mechanism insights.
Our strategies
- Understanding the water oxidation mechanism on iridium oxides
- Developing active and stable non-noble catalysts under acidic condition
- Stability studies and linking the gap between catalysts in lab and industrial PEM electrolysers
 State-of-the-art water electrolysis technology uses an acidic proton exchange membrane as the electrolyte, requiring the use of iridium to catalyze the oxygen evolution reaction (OER) for its incomparable stability in acid. Because of the scarcity of iridium, there is an urgent need to reduce its loading while maintaining activity and stability. Understanding the reaction mechanism and the species governing activity and stability of iridium oxides during water oxidation will enable a better design of active iridium catalysts with low iridium content. Our group aims at combining a series of operando techniques to unravel the fundamental origin of activity and stability of iridium oxides with various structures. We use spectroelectrochemistry (UV-vis, Raman, and X-ray absorption) to probe intermediate states during water oxidation and use mass spectrometry (electrochemistry - mass spectrometry: EC-MS) and time of flight secondary ion mass spectrometry (TOF-SIMS) to quantify oxygen product and proton penetration. Our work on iridium oxides involves close collaboration with Professor James Durrant's group (Imperial College London) and Professor Jan Rossmeisl's group (University of Copenhagen).
State-of-the-art water electrolysis technology uses an acidic proton exchange membrane as the electrolyte, requiring the use of iridium to catalyze the oxygen evolution reaction (OER) for its incomparable stability in acid. Because of the scarcity of iridium, there is an urgent need to reduce its loading while maintaining activity and stability. Understanding the reaction mechanism and the species governing activity and stability of iridium oxides during water oxidation will enable a better design of active iridium catalysts with low iridium content. Our group aims at combining a series of operando techniques to unravel the fundamental origin of activity and stability of iridium oxides with various structures. We use spectroelectrochemistry (UV-vis, Raman, and X-ray absorption) to probe intermediate states during water oxidation and use mass spectrometry (electrochemistry - mass spectrometry: EC-MS) and time of flight secondary ion mass spectrometry (TOF-SIMS) to quantify oxygen product and proton penetration. Our work on iridium oxides involves close collaboration with Professor James Durrant's group (Imperial College London) and Professor Jan Rossmeisl's group (University of Copenhagen).
A major materials bottleneck in the green energy transformation looms in the requirement of iridium to oxidize water at the anodes in polymer electrolyte membrane (PEM) water electrolysis cells. Iridium, a rare element, is not available worldwide at the scale needed for the coming power-to-fuels demand. The challenge of finding a replacement is that most solid materials corrode under the conditions at the PEM anode: low pH and electrochemical potential exceeding 1.5 V vs the reversible hydrogen electrode. A handful of other elements including titanium, manganese, germanium, tin, platinum, and lead are stable, but not active, under typical PEM anodic conditions. By starting with the stable and abundant elements and synthesizing intermetallic oxides, we establish a composition space in which to search for active electrocatalysts. The goal is a material that is stable, conductive, and active – but materials that are stable and conductive only are also of interest as PEM anode supports. Synthesis techniques include magnetron sputter deposition, electrodeposition, and chemical vapor deposition. Novel materials are characterized ex-situ by grazing-incidence x-ray diffraction (XRD), x-ray photoelectron spectroscopy (XPS); and investigated in PEM conditions with EC-MS, ICP-MS, and spectroelectrochemistry. We collaborate with the computational group of Aron Walsh to screen candidate materials.
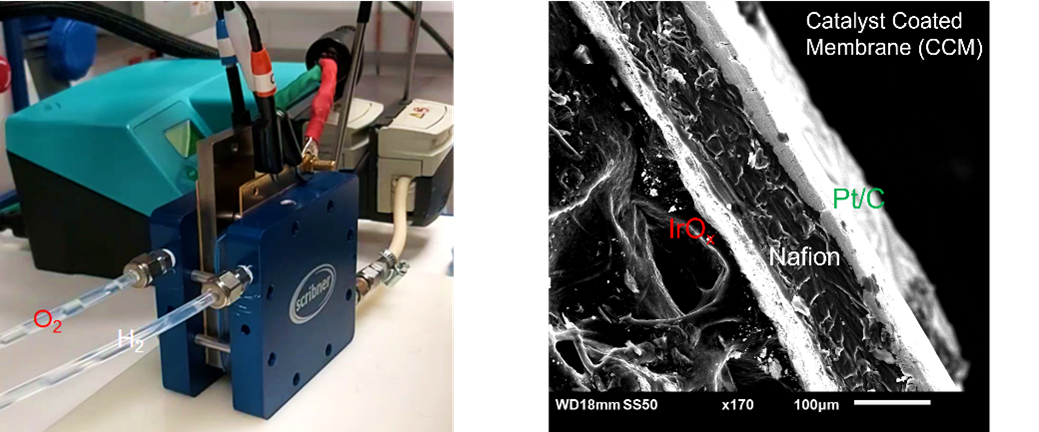
In order to assess the suitability of catalysts for acid-based water electrolysis, both of IrOx and next-generation OER catalysts, suitable testing is required to assess not just the activity of the catalyst but the durability of the material. High stability is required in order to minimise the anode catalyst loading while maintaining an overall lifetime as the catalyst will be exposed to both high potential and acidic environments, alongside a variety of start-up and shutdown procedures that are expected to accompany the use of intermittent energy sources. In order to demonstrate trends at the lab level accelerated stress tests (AST) alongside complementary techniques to monitor catalyst dissolution (ICP-MS) are required to determine the suitability of candidate catalysts to be taken to the next level of testing. Small-scale lab-based testing (RDE) alone is insufficient for predicting catalyst lifetime in a real system due to a variety of differences in both the physical conditions and experimental setup that can result in artifacts in lab-scale testing that are either not present/present to the same extent as in full cell testing (MEA). Understanding this difference and testing catalysts in conditions as close to real operation as possible is key to developing the next generation of OER anode catalysts. We work with both Johnson Matthey and the National Physical Laboratory for full cell testing.