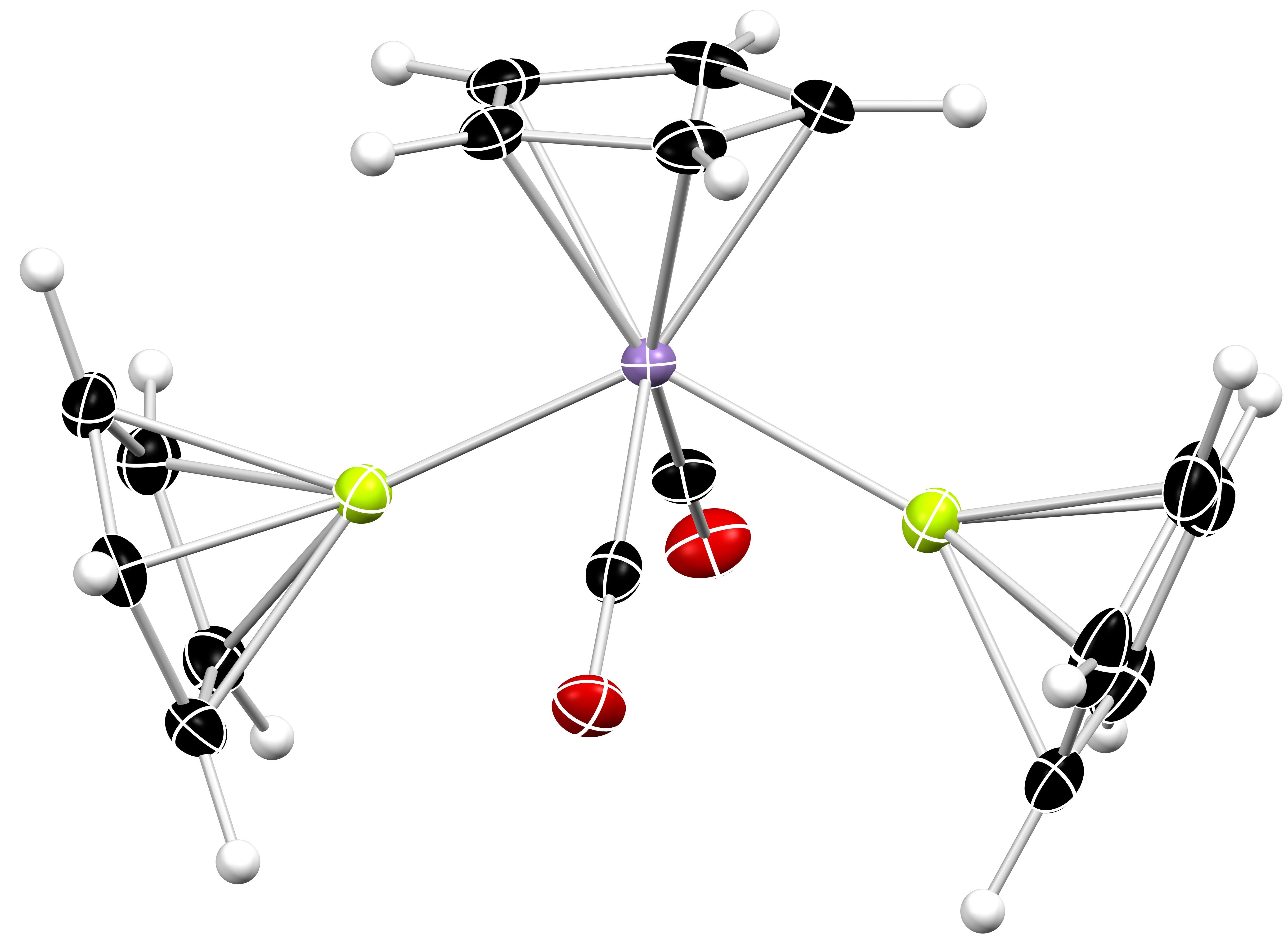
 Metal-Metal Bonding
Metal-Metal Bonding
Classical models of chemical bonding often fail to describe the true nature of an interaction between two (or more) metal atoms. A greater appreciation of the intricacies of metal-metal bonding is, therefore, an important fundamental pursuit. However, molecules featuring metal-metal bonds are also powerful tools; they can be considered soluble “molecular fragments” of bulk metal and can be conveniently probed by a plethora of analytical techniques that cannot be applied to solid materials. So, the targeted study of “exotic” metal-metal bonds can afford wide-ranging insights into the properties of some of the Periodic Table’s most unloved and unusual elements.
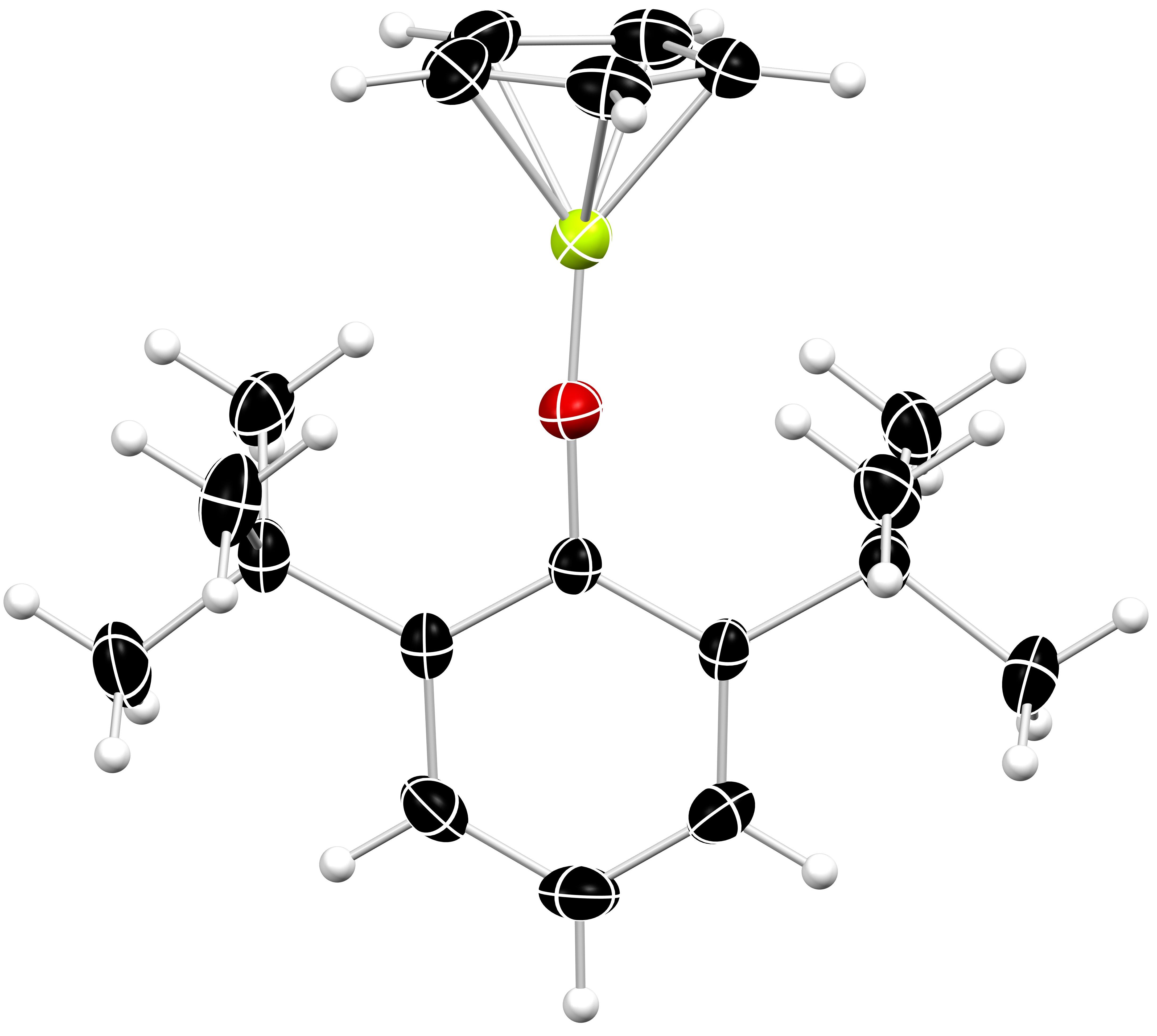 Alkaline Earth Metal Chemistry
Alkaline Earth Metal Chemistry
We are interested in the organometallic chemistry of alkaline earth elements, in particular beryllium and magnesium. Beryllium is the Periodic Table’s fourth element and is unique: it is the smallest metal, exhibits unsurpassed Lewis acidity, and forms chemical bonds with a remarkable degree of covalent character. Yet, owing to its toxicity, beryllium’s chemistry remains almost entirely unexplored. Nonetheless, because the properties of the lightest elements underpin many of our bonding models, a comprehensive understanding of beryllium’s chemistry is of great fundamental importance.
Magnesium is one of the most abundant elements in the Earth’s crust. It is cheap and non-toxic. Thus, the use of magnesium-containing complexes to achieve challenging, industrially relevant chemical transformations is an attractive prospect. Additionally, magnesium organometallics (Gignard reagents) are important laboratory chemicals, used extensively in research and industrial chemistry. So, there are many good reasons to get to know this element better.