Chemical synthesis
Enabling synthetic chemistry underpins all the ongoing research in the group. The motivation of such studies is most often inspired by a desire to pursue functionally important target molecules for a range of applications; from medicinal chemistry and chemical biology to chiral materials chemistry.
Chemical Synthesis
Target-based synthesis
Targets for synthetic chemistry selected by our group are linked to our desire to employ such molecules in a range of multidisciplinary applications. Selected target classes include:
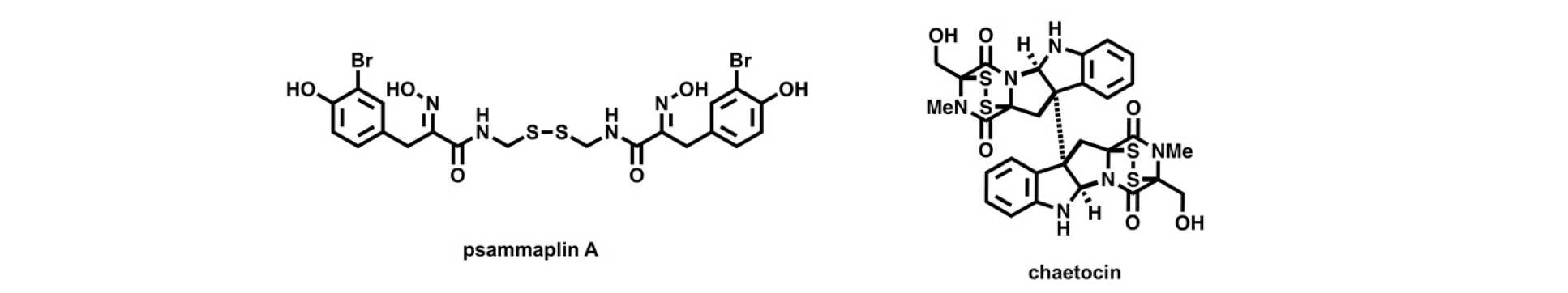
Natural products. Natural products continue to inspire synthetic chemists, often due to their molecular complexity and the challenges this presents for regio-, chemo- and stereo-selective bond construction. Furthermore, naturally isolated small molecules remain "privileged” in their broad range of biological activities. Research in the group continues around the total and/or semi-synthesis of a variety of natural products, linked to our medicinal chemistry and chemical biological studies. Previous targets of the group include psammaplin A and chaetocin, which have been reported to possess key activity against enzymes involved in epigenetic gene regulation. Representative publications: Nat. Prod. Rep. 2013, 30, 605. DOI; Nature Chem. Biol. 2013, 9, 136. DOI; J. Med Chem. 2012, 55, 1731. DOI.
Synthetic targets. Linked to our interest in helical aromatics, conformationally interesting (and often chiral) conjugated systems are key targets for the group. We have published novel methods to control/install a number of forms of molecular chirality including point, axial, and helical chirality. Indeed, while selective methods to create or control single centres displaying point chirality are widespread, effective means of controlling planar, axial and helical chirality are underdeveloped. Representative publications Synlett 2013 24, 2365. DOI Org. Lett. 2013 15, 1706. DOI.
Synthetic methodology
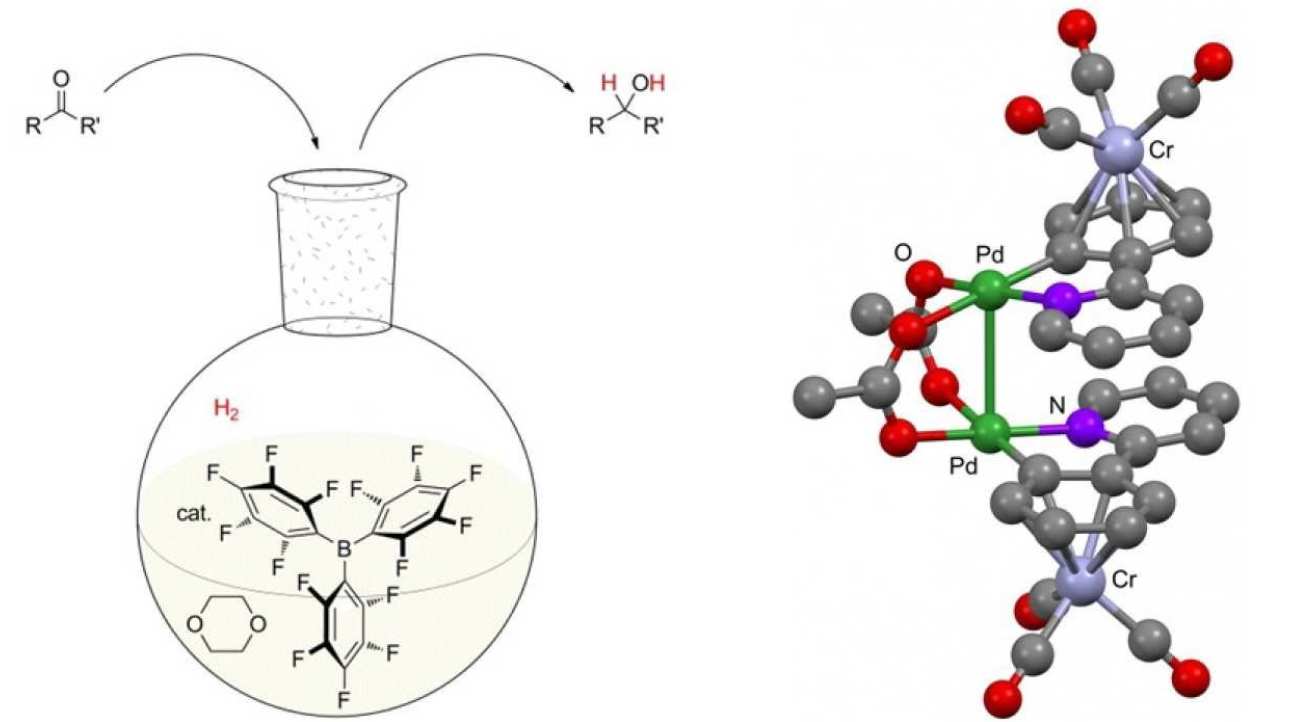
'Frustrated' Lewis pairs. It has recently been discovered that the highly energetically favourable interaction between certain Lewis bases and Lewis acids can be suppressed by increasing the bulk of these molecules. The resulting “frustrated” Lewis pair (FLP), while unable to interact directly, displays fascinating reactivity towards relatively inert molecules/functionality including hydrogen, carbon dioxide, alkenes and alkynes. Together with Dr Andrew Ashley, we have been developing novel methodology involving FLPs catalysis. We have shown that THF solutions of a range of boranes are capable of effecting hydrogen activation in the absence of any additional Lewis base. We have then used this knowledge in the development of the first catalytic and non-metal mediated reduction of C=O bonds using FLPs. Furthermore, we have developed tin-based Lewis acids, which act as a surrogate for the trialkylstannylium ion R3Sn+, and are competent FLP components in the hydrogenation of a number of different unsaturated functional groups with remarkable tolerance to moisture. Ongoing work is focused on kinetic analysis of these catalytic processes and in the development of asymmetric variants. Representative publications: J. Am. Chem. Soc. 2014, 136, 15813. DOI; Angew. Chem. Int. Ed 2014, 53, 10218. DOI; Angew. Chem. Int. Ed. 2016, 55, 14738. DOI; Chem. Soc. Rev. 2017, 46, 5689. DOI.
Transition metal-based methodology. Transition metal mediated catalysis remains a highly important means to carry out a range of selective bond forming processes and we continue to develop new methods and approaches in such reactions. For example, coupled to our interests in helicenes, arylation methodology has been an active area of research for the group. Highlights include the development of palladium and copper mediated processes for the construction of highly bulky aryl-aryl bonds, and the use of arene-π coordination to activate aromatics toward C-H activation. The operationally simple complexation of a chromium tricarbonyl-fragment to an arene ring has drawn much attention in synthetic chemistry, due to the significant change in reactivity and stereochemistry. We have shown that differentially substituted chromium tricarbonyl--complexed aryl pyridines undergo palladation and subsequent arylation using boronic acid nucleophiles. The positioning of the aryl substituents is key in governing substrate reactivity, in order to allow for the geometry required for cyclometallation. Representative publications: Org. Lett. 2013, 15, 1706. DOI; Dalton Trans. 2013, 42, 5615. DOI; Org. Biomol. Chem. 2013, 11, 31. DOI.


