Electrochemical nitrogen reduction to ammonia is a sustainable alternative to the fossil-fuel sourced, energy-intense, and centralised Haber-Bosch process - currently responsible for >1% of our global greenhouse gas emissions. By harvesting renewable electricity and operating under ambient conditions, this more sustainable route offers local and dynamic production of ammonia: the precursor for fertilisers, a potential marine fuel, and the inevitable building block of most N-containing chemicals in industry. However, nitrogen reduction is highly challenging since splitting N2 requires a lot of energy, and the much easier hydrogen evolution - the reduction of protons to hydrogen gas - is prevalent.
State of research
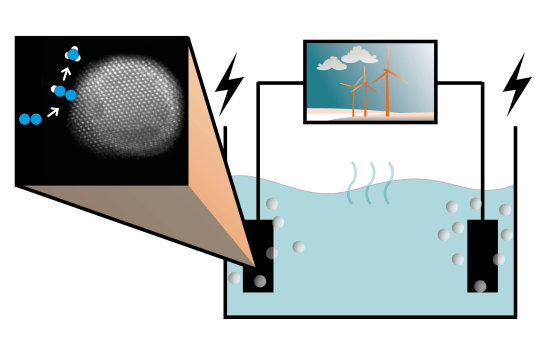 To date, the only electrochemical system capable of unambiguous conversion of nitrogen gas to ammonia in significant amounts and with tangible efficiency is a metal-mediated nitrogen reduction,1,2 using lithium/calcium as a redox mediator in a non-aqueous electrolyte to provide a ground for high selectivity against side-reactions such as hydrogen evolution. The electrolyte used in this lithium-mediated process is analogous to battery electrolytes. Just like in batteries, a passivating layer builds up on the negative electrode upon application of a voltage. This phenomenon - occurring during nitrogen reduction too - is likely to play a major role in its performance but remains a largely unexplored aspect of the system.3
To date, the only electrochemical system capable of unambiguous conversion of nitrogen gas to ammonia in significant amounts and with tangible efficiency is a metal-mediated nitrogen reduction,1,2 using lithium/calcium as a redox mediator in a non-aqueous electrolyte to provide a ground for high selectivity against side-reactions such as hydrogen evolution. The electrolyte used in this lithium-mediated process is analogous to battery electrolytes. Just like in batteries, a passivating layer builds up on the negative electrode upon application of a voltage. This phenomenon - occurring during nitrogen reduction too - is likely to play a major role in its performance but remains a largely unexplored aspect of the system.3
Our strategy
We currently work towards characterising the electrolyte as well as the reactive interface of the Li-mediated nitrogen reduction via various high-end ex-situ, in-situ and operando techniques. By coupling electrochemical measurements with in-line interface and bulk analysis, we hope to uncover the underlying mechanisms governing the bulk and interfacial processes occurring over the course of the electrolysis, the passivating layer composition, its behaviour, and the ensuing activity, stability, and selectivity of the systems under study, towards ammonia synthesis.
References
- Andersen, Čolić, Yang, Schwalbe, Nielander, McEnaney, Enemark-Rasmussen, Baker, Singh, Rohr, Statt, Blair, Mezzavilla, Kibsgaard, Vesborg, Cargnello, Bent, Jaramillo, Stephens, Nørskov, Chorkendorff, A rigorous electrochemical ammonia synthesis protocol with quantitative isotope measurements, Nature 2019, 570, 504–508. https://www.nature.com/articles/s41586-019-1260-x
- Fu, Niemann, Zhou, Li, Zhang, Pedersen, Saccoccio, Andersen, Enemark-Rasmussen, Benedek, Xu, Deissler, Mygind, Nielander, Kibsgaard, Vesborg, Nørskov, Jaramillo, Chorkendorff, Calcium-mediated nitrogen reduction for
electrochemical ammonia synthesis, Nature Materials 2024, 23, 101–107. https://www.nature.com/articles/s41563-023-01702-1 - Westhead, Barrio, Bagger, Murray, Rossmeisl, Titirici, Jervis, Fantuzzi, Ashley, Stephens, Near ambient N2 fixation on solid electrodes versus enzymes and homogeneous catalysts, Nature Reviews Chemistry 2023, https://doi.org/10.1038/s41570-023-00462-5