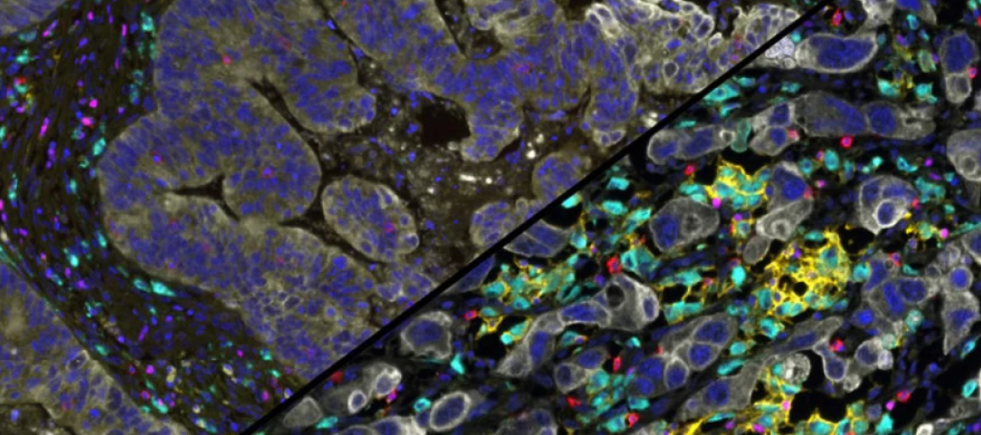
What we do
Our group is interested in the identification of diagnostic and prognostic biomarkers and potential therapeutic targets for several types of tumours and in particular ovarian tumours. Ovarian neoplasms are principally classified into epithelial tumours, sex cord stromal tumours, and germ cell tumours. Each group includes benign and malignant neoplasms and there are also tumours of uncertain malignant potential and borderline tumours. These tumours are encountered at different frequencies in clinical practice, with a largely proportionate inclusion in clinical and basic science studies.
We have a special interest in borderline ovarian tumours and sex cord stromal tumours. Published work from our laboratory shows that there are molecular subtypes of serous borderline ovarian tumours with benign like and malignant like signatures. Continued work in this area will hopefully lead to the identification of predictive biomarkers for the biological behaviour of individual borderline tumours, as well as the identification of potential therapeutic targets for tumours showing aggressive behaviour; both of which remain to be unmet clinical needs. Similar challenges are also encountered in the management of some sex cord stromal tumours in addition to the need for the identification of robust diagnostic biomarkers for rare entities within this group, particularly ones that present with atypical histological features.
To explore this we analyse patient samples using a range of advanced techniques as well as techniques used in diagnostic histopathology laboratories to study the translational potential of our findings.
Our laboratory is privileged by always working on the basis of close collaboration between scientists and clinicians. This is very much embodied in our work on borderline ovarian tumours where we have been working for at least 10 years now in collaboration with clinical teams in several centres in the United Kingdom through a National multicentre study led by our group, in addition to our work with our colleagues in the multidisciplinary team of the West London Gynaecological Cancer Centre based at the Hammersmith and Queen Charlotte and Chelsea Hospitals. The aim of our work is to develop protocols for optimal care for patients with borderline ovarian tumours from primary diagnosis to personalised surgical procedures and follow-up plans and management of progressive disease.
Why it is important
Epithelial ovarian tumours are the commonest types of ovarian tumours and they affect women of different age groups, including young patients in the reproductive age group. Early diagnosis, precise tumour typing and stratification in terms of prognosis and eligibility to generic and targeted therapy are important aspects for tumour management.
How it can benefit patients
Borderline ovarian tumours usually affect women in the reproductive age group, and carry the potential for recurrence and progression to cancers that can happen after many years. Their management ideally requires radical surgery and long term follow up, both of which have serious effects on these young women in terms of potential loss of fertility and long term and health concern related anxiety. The same challenges are also shared by some sex cord stromal tumours.
Both of these entities are relatively understudied in comparison to the commoner and more aggressive types of ovarian tumours. Our work on these entities addresses questions and gaps of knowledge that have the potential to provide findings that contribute to evidence based personalised medical managment for these patients, matching the choice of therapy with what would work best for each individual patient.
Summary of current research
- Exploring the tumour microenvironment of ovarian cancer
- Tumour on chip models for the study of cancer
- Proteomic and metabolomic profiling of fluids from patients with gynaecological tumours
- Impact of gynaecological tumours on fertility
- Survey on fertility preservation in young women with gynaecological tumours
In this project we are exploring the detailed histopathological features and immune landscape of ovarian cancer by assessing the expression of immune checkpoint molecules and their correlations with immune resident cells and cytokine network. We employ different techniques on in vivo and in vitro models.
Why it is important: Comprehensive Knowledge of the tumour microenvironment is essential to understanding histogenesis, signalling pathways and identifying prognostic biomarkers and therapeutic targets.
How it can benefit patients: Identifying immunotherapeutic targets and prognostic biomarkers would be most valuable in developing evidence based personalised treatment plans for ovarian cancer patients.
Tumour-on-a-chip models are a very promising approach for studying cancer development, metastasis, and treatment strategies. We are reviewing the strengths and limitations of currently available models, with a focus on their application to ovarian cancer.
Why it is important: Having a comprehensive understanding of the current state of tumour-on-a-chip technology provides the foundation for developing novel designs to use in our studies on ovarian cancer, replicating different aspects of the tumour microenvironment.
How it can benefit patients: Working with our collaborators in the Faculty of Engineering we aim to develop models with potential for use in clinical practice for drug screening to identify individual tumour responses to different therapeutic agents for personalised patient treatment.
Body fluids from patients with ovarian tumours may contain tumour related biomarkers which can be detected by proteomic and metabonomic profiling.
Why it is important: A prominent issue in the treatment of ovarian tumours is that the current diagnostic measures are not all suited for early diagnosis, resulting in poorer patient outcomes. Proteomic and metabonomic profiling of fluids has the potential of providing tumour related signatures with diagnostic potential.
How it can benefit patients: The identified biomarkers have great potential value for early diagnosis and follow up of patients with ovarian tumours.
Many factors can affect young women’s fertility, including being diagnosed with a gynaecological tumour. In this project we explore the impact of gynaecological tumours on ovarian follicular status.
Why it is important: This study seeks to uncover how these tumours affect the health and quality of ovarian follicles, with the potential to identify biomarkers that could predict ova quality.
How it can benefit patients: Understanding these biological changes offers foundations for more effective fertility preservation strategies, which could have significant implications for improving fertility outcomes in affected women.
We are running a survey addressed to young women diagnosed with gynaecological cancer and borderline ovarian tumours.
Why it is important: The survey will provide valuable insights into patients’ understanding of fertility preservation, their concerns, and the factors that influence their treatment choices.
How it can benefit patients: By identifying gaps in knowledge and understanding patient priorities, the study will provide foundations for enhancing support, counselling, and decision-making tools development for women facing these challenges.
Information
Funders
- Imperial BRC Funding
- North West London Pathology
- Egyptian Ministry of Higher Education
- Saudi Arabia Government Scholarships
Related centres
- West London Gynaecological Cancer Centre
- Wolfson Fertility Centre
- MRC-NIHR BRC National Phenome Centre
- Imperial College Healthcare NHS Trust Tissue Bank
- Institute of Cancer Research – Tumour Profiling Unit
Internal
- Dr Ernesto Yague, Department of Surgery and Cancer
- Prof Christina Fotopoulou, Department of Surgery and Cancer
- Dr Joseph Yazbek, Department of Surgery and Cancer
- Prof Iain McNeish, Department of Surgery and Cancer
- Prof Sadaf Ghaem-Maghami
- Prof Maria Kyrgiou
- Mr Srdjan Saso
- Dr Jia Li
- Mr Sotirios Saravelos
- Prof Darryl Overby
- Prof Ferdinando Rodriguez y Baena
- Mr Mathias Winkler
- Mr Martin J Connor
External
Find all of Professor Mona El-Bahrawy's publications here.
- Professor El-Bahrawy was the lead investigator of the multicentre study, Borderline ovarian tumours: A strategy for developing optimal care.
- Professor El-Bahrawy was the nominated pathologist at Imperial College London for the following trials:
- A Two-Part, Phase I Open Label, Dose Escalation Study To Assess The Safety, Pharmacokinetics And Clinical Activity Of Nuc-1031, A Nucleoside Analogue, In Participants With Advanced Solid Tumours.
- A Two-Part Phase 1/2a, Open-Label, Dose-Escalation Study to Evaluate the Tolerability and Preliminary Antitumour Activity of OPB-111001 in Patients with Advanced Cancers that are Poorly Responsive to Standard Anticancer Treatment.
- A Phase 1b, multi-center, open-label, dose escalation study of GSK2256098 (FAK inhibitor) in combination with Trametinib (MEK inhibitor) in subjects with advanced solid tumours.
- A Phase I Open-Label Dose-Escalation Study of the Focal Adhesion Kinase Inhibitor, GSK2256098, in Subjects with Solid Tumours.
- SCOTROC 4: A prospective, multicentre, randomized trial of carboplatin flat dosing Vs intrapatient dose escalation in first line chemotherapy of ovarian, fallopian tube and primary peritoneal cancers.
- An Open Label Study To Investigate the Pharmacokinetics and Pharmacodynamics of Repeat Escalating Doses of the Oral AKT Inhibitor GSK2141795 by 18F FDG PET Analysis in Subjects with Ovarian Cancer.
- Phase I, open-label, multi-center, dose-escalation study with extension to evaluate safety, pharmacokinetics and activity of CH5132799, a PI3K inhibitor administered orally as a monotherapy in patients with advanced solid tumors.
- A phase 1 open-label, dose-finding study to evaluate the safety and pharmacokinetics of ONX 0801, a novel α-folate receptor-mediated thymidylate synthase inhibitor, in patients with advanced solid tumours.
- A phase 2, open-label test-retest study to assess the reproducibility of quantitative measurements of 18F uptake by solid tumours using pet imaging following intravenous administration of AH111585 (18F) injection.
- [18F]fluorothymidine-positron emission tomography (FLT-PET) and DCE/DW-MRI for early response monitoring in epithelial ovarian cancer (EOC).
- mEOC: A GCIG Intergroup multicentre trial of open label carboplatin and paclitaxel +/- bevacizumab compared with oxaliplatin and capecitabine +/- bevacizumab as first line chemotherapy in patients with mucinous Epithelial Ovarian Cancer (mEOC).
- Randomised Phase III Trial of Paclitxel plus Carboplatin (TC) Therapy versus Irinotecan plus Cisplatin (CPT-P) Therapy as a First Line Chemotherapy for Clear Cell Carcinoma of the Ovary.
Our researchers
Professor Mona El-Bahrawy
/prod01/channel_3/media/images/people-list/El-Bahrawy,_Mona_2015--tojpeg_1545048174295_x1.jpeg)
Professor Mona El-Bahrawy
Professor of Practice (Histopathology)
Lamiaa Sabry
/prod01/channel_3/media/images/people-list/Lamiaa-Sabry.jpg)
Lamiaa Sabry
PhD student
Sarah Gouharji
/prod01/channel_3/media/images/people-list/image0.jpeg)
Sarah Gouharji
PhD student
Nanki Deol
/prod01/channel_3/media/images/people-list/Nanki-Deol.jpg)
Nanki Deol
MRes Cancer Biology student
Related courses
MRes Cancer Biology
MRes Biomedical Research
