Against the clock: uncovering the first 30 minutes of viral infection
by Rachel Kahn
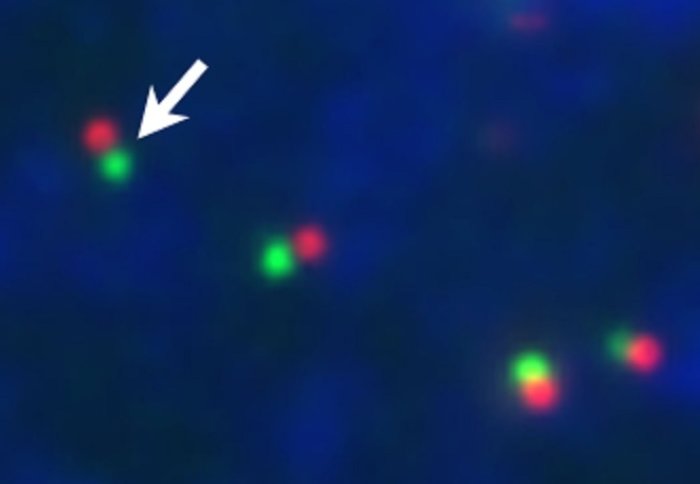
Researchers are unraveling the crucial first 30 minutes of viral invasion into a human cell.
For a virus attacking a cell, timing is everything. In order to take hold, these invaders must act efficiently, avoiding immune detection and remaining intact whilst they journey through cellular machinery.
However, the intricacies of the early stages of viral life have for years troubled scientists who have been studying these small but detailed particles. Understanding the mechanisms will hopefully help them create new therapeutics for viral diseases.
Work from one team at Imperial is now shining more light on the earliest stages of viral infection, helping to answer some of the questions about what happens once these microbial invaders enter the host cell.
Peter O’Hare, Professor of Virology from the Department of Medicine, leads a group looking into what happens when our cells come under attack from a common family of viruses implicated in everything from cold sores to cancer – the Herpesviruses.
By studying one member of this class of virus (HSV), they are beginning to unravel questions about how viruses get into the host cell and travel to the nucleus, where they hijack the cell’s machinery to produce copies of themselves, and how they regulate elements of the host cell required for infection. We asked him more about his latest research.
Why are the first 30 minutes of infection so important?
Knowing what a virus does in the first 30 minutes, and exactly how the viral genome – the virus’s genetic material – gets into the cell is critical. We can then study which cellular proteins are used by the virus, and the processes by which it manages to evade any immune response.
If we know about these processes, we can rationally design compounds that interfere with them, and these could make good potential therapeutics for viral diseases.
What does the virus do when it finds and enters a host cell?
Like many other viruses, HSV has to find its way to the nuclear pore – a sort of gateway that allows entry into the nucleus of the cell it’s infecting. It has a certain time frame to do this before ‘alarm bells’ start ringing and a host cellular response is triggered to stop its entry.
Because of this, viruses have evolved specific ways to fast-track themselves through the host cell, and we identified a small section of a protein that, if absent, would mean HSV would lose its ability to target itself and enter through the nuclear pore.
HSV does this very efficiently, and we also found that the viral genome is held together very tightly and remains intact in the host cell’s cytoplasm. This is interesting as it means the host cell cannot detect viral DNA ‘hiding’ in the cytoplasm and therefore won’t be able to trigger an immune response.
This might not be the same in all cell types but it’s certainly an interesting starting point.
In the past, available techniques have made it difficult to trace and measure viral genomes. How did your team manage to do this?
Over the last few years, chemists have been able to take small chemical groups and tag them onto biological molecules, which are precursors for other molecules, like DNA and proteins.
We are using one of these DNA precursors to feed into cells. We can then infect those cells with HSV in the hope that when the virus replicates its DNA, it will take the DNA precursor that we chemically tagged with it. From there, we can purify out the virus and use it to infect other host cells.
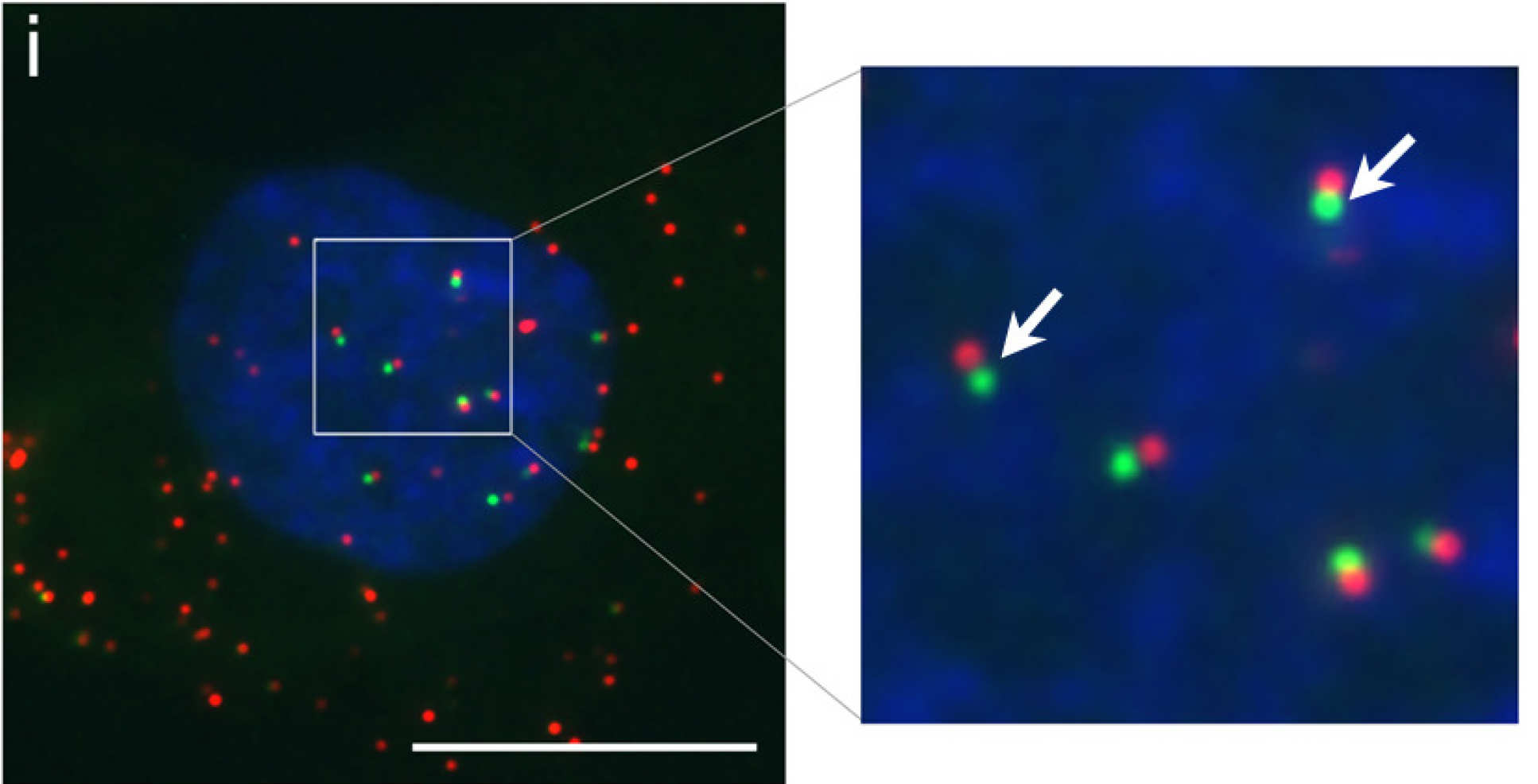
The great thing is, we can chemically tag the DNA precursors with a fluorescent molecule, which means when it is attached to the viral genome it will fluoresce, allowing us to detect it and visualise how it infects a cell. So we have now, for the first time, visualised the first 30 minutes of infection and observed the genome traversing across the nuclear pore gateway. There is great excitement over this technology and what it could promise for the future.
What could this new information mean for the future?
We’re interested in the long term and this involves understanding the mechanism of an event so we can interfere with it. Having this knowledge puts us in a position to be able to better design therapeutic interventions and we certainly hope our research can feed into this.
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Rachel Kahn
Communications and Public Affairs