Watching the immune system in action reveals what happens when things goes wrong
by Rachel Kahn
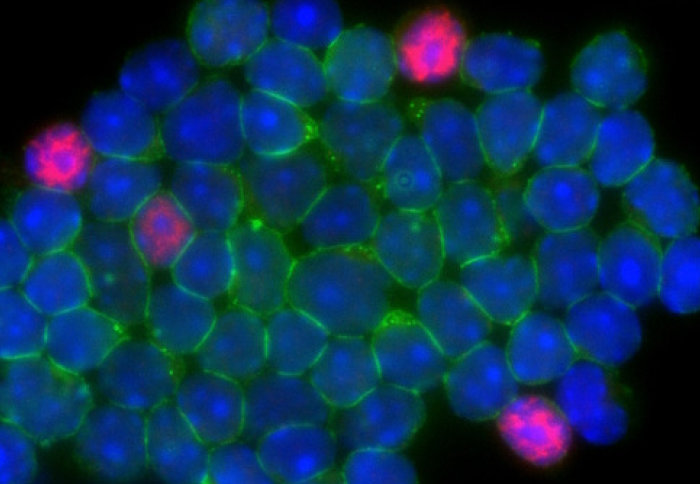
Scientists are unveiling how our immune system works – and malfunctions – thanks to an innovative technology that tracks immune cells.
The technology has already been used to look at immune cells involved in a mouse model of multiple sclerosis, and could provide valuable insights into autoimmune diseases.
What I hope is that by improving our understanding of how cells work systemically in the body, we can create a better and even more tailored method to manipulate our immune system for human health benefits. Dr Masahiro Ono
As immune cells travel and work all around the body, they have been incredibly difficult to track and understand in the past. Now, following a five-year study, researchers at Imperial have developed a technology that could change this.
The immune system is vital in human health and wellbeing, with different types of cells continuously roaming our bodies, primed to fight infection. However, the immune system is associated with a number of diseases, termed ‘autoimmune’ diseases where it turns on our body and starts producing a response where it’s not required.
The research, published today in the Journal of Cell Biology, specifically looked at T cells, a key immune cell in the fight to clear infection.
Lead author Dr Masahiro Ono, a BBSRC fellow from the Department of Life Sciences at Imperial, said: “This new method allows us to identify and analyse the activities of cells inside the body in a systemic manner, giving us a better understanding of how they work over time.”
Seeing infection responses in action
In response to infection, T cells are activated through a marker on their surface known as the T cell receptor (TCR), which is the eyes of the cell. When the TCR 'sees' an invading pathogen, it triggers a signal telling the cell to turn, or ‘differentiate’, into a type of T cell able to carry out a specific task in the fight against infection.
Using mice, Dr Ono and his team were successfully able to characterise how a T cell activates and differentiates over time. This process of differentiation has been previously difficult to characterise due to the very dynamic nature of the process.
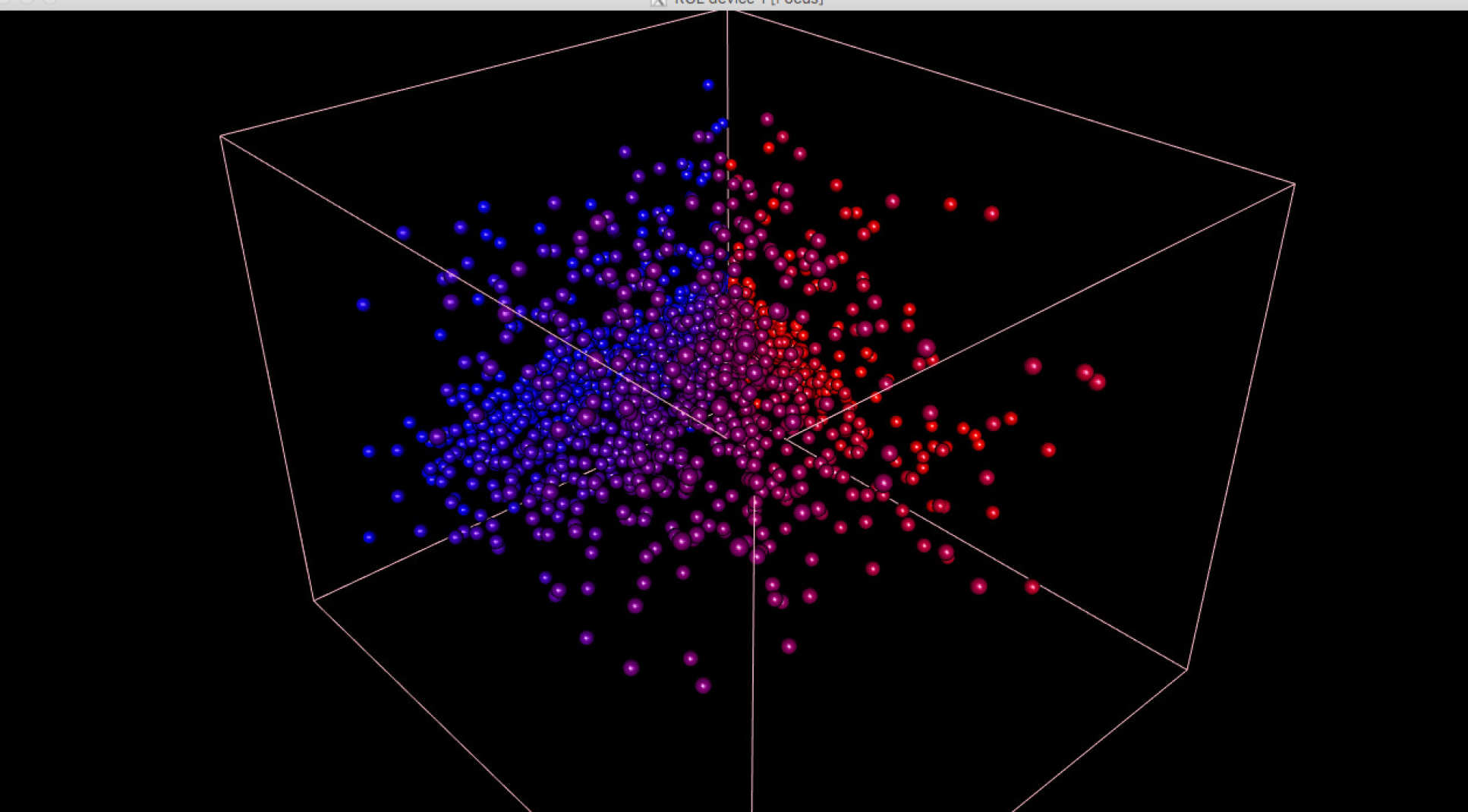
The team developed a new technology known as Timer-of-cell-kinetics-and-activity or, ‘Tocky’, after the Japanese word for time, where a fluorescent protein known as Timer is attached to a gene involved in T cell function.
By adding the Timer protein, which changes colour from blue to red as T cells are activated, the team could identify the events going on inside the cell across time as activation occurred, including identifying which signalling pathways were triggered.
Dr Ono added: “Using this new technology, we were able to understand and correlate events with the passage of time following T cell receptor signalling in vivo. This has been previously very difficult to do.”
Watching when T cells attack
Additionally, the team were able to identify pathogenic (disease-causing) T cells in a mouse model of multiple sclerosis, a disorder where immune cells, including T cells, attack the brain and spinal cord.
As humans we have 100 million T cells in our body at once, making it challenging to look at which T cells carry out specific tasks. However, using this new technology, the team were able to show that pathogenic T cells involved in multiple sclerosis have very unique activities in the brain and spinal cord across time, interacting with specific proteins and being continuously reactivated.
This knowledge could help scientists to further understand the disease and potentially come up with new treatment possibilities in the future.
Additionally, many immunotherapies, used to treat certain cancers for example, target T cells, but how our immune system responds to these therapies can be difficult to predict.
Dr Ono said: “What I hope is that by improving our understanding of how cells work systemically in the body, we can create a better and even more tailored method to manipulate our immune system for human health benefits.”
The next steps for Dr Ono and his team will be to try and translate this new technology so it works for other cell types, giving us a deeper understanding of how our cells work systemically around our bodies.
-
'A timer for analyzing temporally dynamic changes in transcription during differentiation in vivo' by David Bending, Paz Prieto Martín, Alina Paduraru, Catherine Ducker, Erik Marzaganov, Marie Laviron, Satsuki Kitano, Hitoshi Miyachi, Tessa Crompton, and Masahiro Ono is published in Journal of Cell Biology.
Article supporters
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Rachel Kahn
Communications and Public Affairs