How structures of complement complexes guide therapeutic design
Congratulations to Dr Jasmine Bickel on the publication of a book chapter in the Subcellular Biochemistry book series.
The chapter ‘How Structures of Complement Complexes Guide Therapeutic Design’ forms part of the book Macromolecular Protein Complexes III: Structure and Function and was written by EPSRC Doctoral Prize fellow Dr Jasmine Bickel in collaboration with PhD student Tomas Voisin, Professor Ed Tate and Dr Doryen Bubeck (Life Sciences). Jasmine completed her PhD in 2020 as part of the Tate and Bubeck labs and is currently carrying out her fellowship in the lab of Professor Ian McNeish in the Department of Surgery and Cancer.
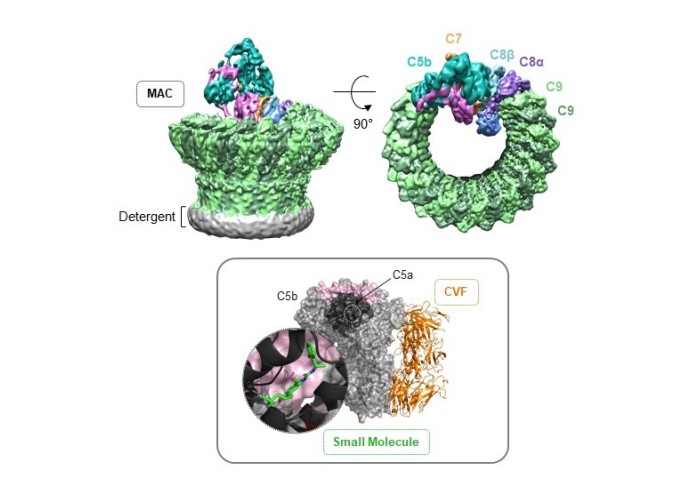
The complement system is one of the body’s main mechanisms to trigger an immune response and is essential for defence against infection and regulation of this response. Activation of the complement pathway leads to formation of the membrane attack complex (MAC), a multiprotein pore that punctures membranes. The chapter reviews the structural basis for MAC formation with particular focus on information gained from recent advances in cryo-electron microscopy (cryoEM) - a technique that allows high resolution structures to be generated without the need for crystallisation and the subject of the 2017 Nobel Prize in Chemistry. The chapter concludes with discussion of the regulatory complexes involved in MAC formation and their impact on structure-guided drug discovery of complement therapeutics.
Congratulations Jasmine!
Article supporters
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Jennie Hutton
Department of Chemistry