NMR Centre identifies a new molecular linkage in body’s energy stores

Using nuclear magnetic resonance, researchers have identified a new ubiquitin-polysaccharide linkage on glycogen.
The results, published today in EMBO Journal, are the first to describe the attachment of ubiquitin to sugars, rather than proteins, and to suggest the role this linkage plays in healthy cells.
Understanding its function could also help understand what happens when the process goes wrong – leading to a build-up of sugars that can damage organs, including the heart.
Our combined experience and knowledge in applying NMR to these systems has uncovered a new molecular species in biochemistry. Professor Steve Matthews
Ubiquitin is a ubiquitous protein found in all plant, fungi, and animal cells, which has remained virtually unchanged by evolution. It can be attached to proteins flagging them for removal, relocation, or a change in activity, thereby affecting many basic cellular processes involved in health and disease.
A variety of cellular enzymes are involved in building ubiquitin chains and attaching them to the target - ubiquitylation. HOIL-1 is one such enzyme that, in one of its roles, prevents aberrant accumulation of toxic aggregates of glycogen.
Glycogen is a polymer of the simple sugar glucose that serves as a form of energy storage. Defects in HOIL-1 activity results in accumulation of insoluble starch-like sugar, known as polyglucosan, in cells leading to organ damage. A notable example is the development of cardiomyopathy and death from heart failure in early adulthood.
To clear polyglucosan deposits before its toxic effects are realised, Professor Sir Philip Cohen from the University of Dundee demonstrated that HOIL-1 can not only ubiquitylate proteins but also polyglucosan sugars. Despite the importance if this discovery, the attachment point of ubiquitin on the sugar molecule remained a mystery.
Successful collaboration
A recent successful collaboration with the Cross Faculty NMR Centre at Imperial College London led the Dundee team to Professor Steve Matthews. The combined forces immediately realised that nuclear magnetic resonance (NMR) was an ideal tool to resolve the puzzle of ubiquitin-sugar attachment.
NMR allows the molecular structure of molecule to be analysed by measuring the interaction of nuclear spins when placed in a powerful magnetic field. Using NMR isotope labelling and multi-nucleus methods at high field, a novel linkage was detected between ubiquitin and polymeric glucose substrates.
The team found that one ubiquitin subunit was specifically attached to a single glucose subunit through an ester bond to the C6 side chain carbon of the sugar ring. With this question answered, the way in which polyglucosan could be recognised and ubiquitylated suddenly made sense.
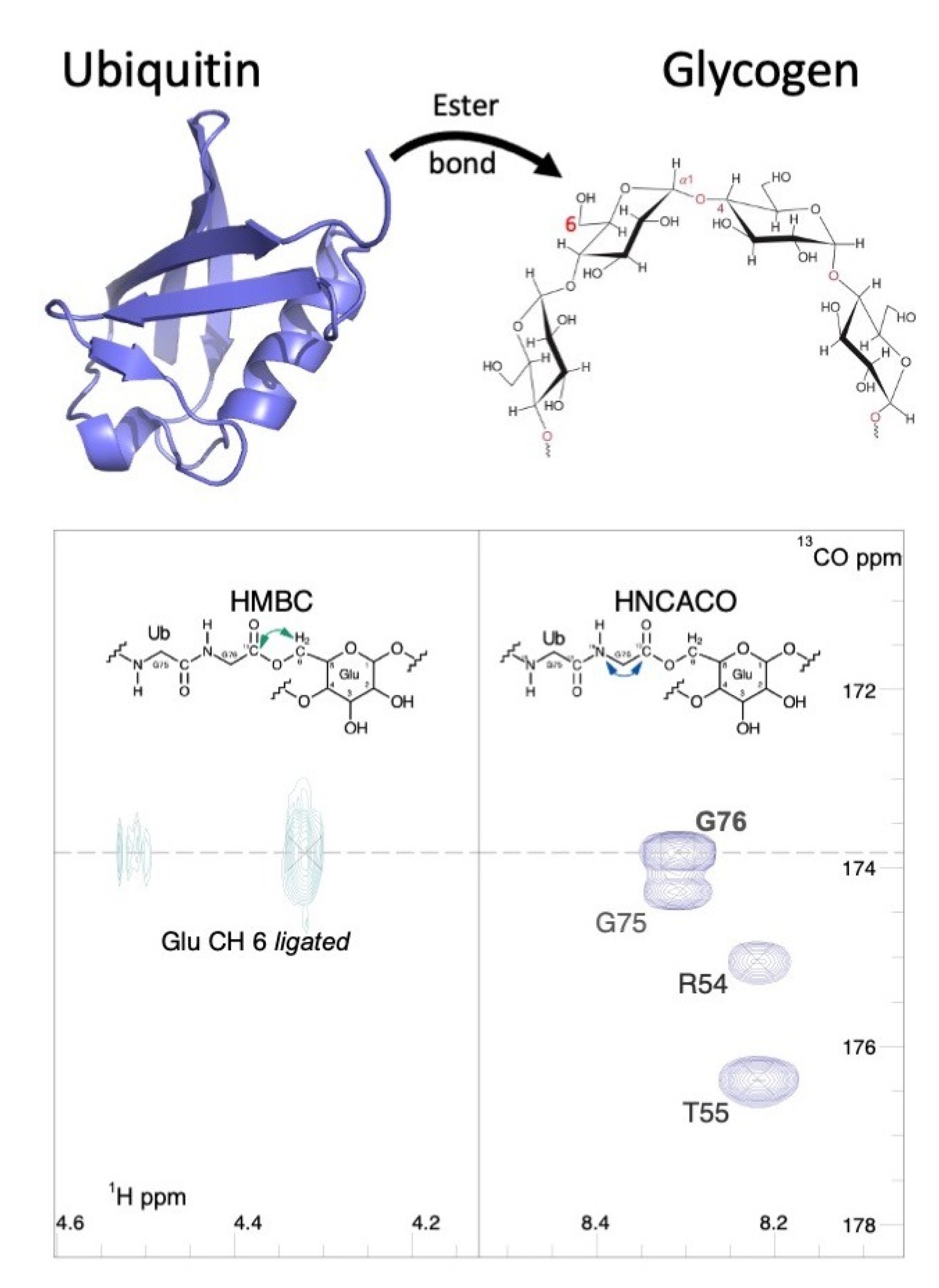
Normal glycogen is heavily branched where new sugar polymers are linked to stem chains, which aids water solubility and efficient utilization when broken down, whereas malformed polyglucosan adopts an unusual structure with fewer branches and is insoluble. The branching point for healthy glycogen is the same C6 carbon position identified by NMR as the ubiquitin attachment site on glucose polymers.
It was therefore concluded that HOIL-1 recognises unbranched glycogen molecules via free glucose C6 side chains and attaches a ubiquitin molecule, which flags it to cellular quality control systems that would remove the abnormal forms of glycogen.
Power of the NMR Centre
These results are the first to describe the ubiquitylation of sugars, identify the point of attachment and suggest a role in healthy cells. Furthermore, ubiquitylation of non-protein biological molecules is likely to be a wider tactic employed by cells to regulate many more biological molecules.
Study author, Professor Steve Matthews, director of the CFNMR Centre, said: “This work highlights the immense power of the NMR spectrum as its properties can be directly related to molecular structure. Our combined experience and knowledge in applying NMR to these systems has uncovered a new molecular species in biochemistry.”
Study author, Dr Yingqi Xu, facility manager of the CFNMR centre, said: “The NMR couplings between sugar and ubiquitin are very small, and their detection was only possible thanks to our high-end NMR equipment with the Centre.”
-
‘HOIL-1-catalysed ubiquitylation of unbranched glucosaccharides and its activation by ubiquitin oligomers,’ by Kelsall IR, McCrory EH, Xu Y, Scudamore CL, Nanda SK, Mancebo-Gamella P, Wood NT, Knebel A, Matthews SJ, Cohen Pclose, is published in EMBO Journal.
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Press Office
Communications and Public Affairs
- Email: press.office@imperial.ac.uk
