Five ways Imperial research is making an impact on our understanding of dementia
To mark Dementia Action Week, we look at how Imperial College London is making an impact on our understanding of dementia.
Dementia Action Week 2022 runs from 16-22 May, focusing on the theme of diagnosis. With more than 900,000 people in the UK living with dementia, research shows that the common belief around memory loss being a sign of normal ageing is the biggest obstacle to people seeking a diagnosis.
This week is an opportunity to raise awareness and encourage those who might be living with undiagnosed dementia to come forward and feel ready to take the next step in seeking support. To mark Dementia Action Week, we look at how Imperial College London has been leading the fight against dementia.
1. We’ve engineered common baker's yeast to produce a key ingredient for dementia medicines
 Earlier this year, researchers from Imperial College London and National University of Singapore (NUS) were able to convert yeast into bio-factories to produce a chemical compound called D-lysergic acid (DLA). DLA is an alkaloid used in medicines to treat dementia and Parkinson’s disease, as well as migraines and other neurological conditions. It is estimated that 10 to 15 tons of the compound are produced each year to meet the global demand for such medications.
Earlier this year, researchers from Imperial College London and National University of Singapore (NUS) were able to convert yeast into bio-factories to produce a chemical compound called D-lysergic acid (DLA). DLA is an alkaloid used in medicines to treat dementia and Parkinson’s disease, as well as migraines and other neurological conditions. It is estimated that 10 to 15 tons of the compound are produced each year to meet the global demand for such medications.
Professor Paul Freemont, from the Department of Infectious Disease at Imperial College London, and co-lead Principal Investigator of the study, said: “Yeast has been a key part of human civilization for thousands of years, helping us to make bread and brew beer. But our relationship with this familiar microbe is evolving. Through this exciting collaboration we have been able to harness fungal cells to act as miniature factories to produce the raw compounds for medicines.”
"This is an example of how something seemingly small and inconsequential has the potential to change human lives, providing the drugs which will enable us to age better and reduce the environmental impact of industrial drug production.”
2. Our researchers are designing molecules which obstruct Alzheimer’s peptides
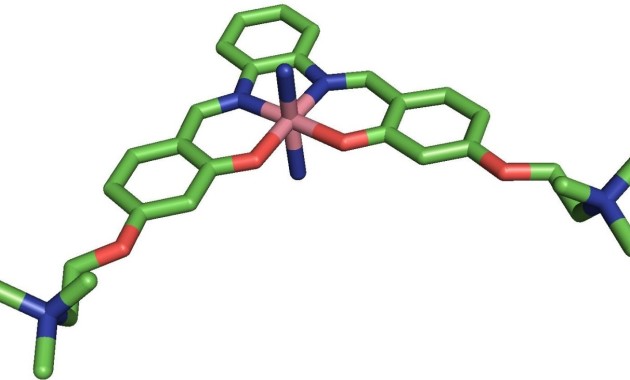 Last summer a team of Imperial researchers designed a metal-based molecule that is highly effective at preventing the build-up of one of the key hallmarks of Alzheimer's disease - a specific peptide known as amyloid-β.
Last summer a team of Imperial researchers designed a metal-based molecule that is highly effective at preventing the build-up of one of the key hallmarks of Alzheimer's disease - a specific peptide known as amyloid-β.
The team demonstrated that with the aid of ultrasound, their molecule can cross the blood-brain barrier in mice, targeting the part of the brain where the damaging peptide most often accumulates.
First author Tiffany Chan, from the Departments of Chemistry and Bioengineering at Imperial, said: “Very few metal-based molecules have been investigated as potential inhibitors of amyloid-β build-up because of toxicity issues and difficulty crossing the blood brain barrier."
"The molecule we have designed is able to interfere with amyloid-β and seems non-toxic, and it can be delivered across the blood brain barrier using ultrasound, which means you don’t need an invasive procedure.”
3. We’re creating an ‘atlas’ of the brain to map different stages of Alzheimer’s disease
The UK Dementia Research Institute's (UK DRI) Multi-‘omics Atlas Project (MAP) was launched in March 2020 as a 2m initiative to use an unprecedented range of advanced techniques to examine tissue from eight different regions of the brain.
 The project was designed so that it can be extended to include new techniques as they are developed, using the same set of donor brains, and to add new data to the existing dataset. It is hoped that the study could eventually be expanded to map the pathology of other neurodegenerative diseases. By studying tissue at the early stage of illness, the team hopes to determine the most optimal time to target the disease before widespread neurodegeneration takes place.
The project was designed so that it can be extended to include new techniques as they are developed, using the same set of donor brains, and to add new data to the existing dataset. It is hoped that the study could eventually be expanded to map the pathology of other neurodegenerative diseases. By studying tissue at the early stage of illness, the team hopes to determine the most optimal time to target the disease before widespread neurodegeneration takes place.
This was the first time that UK brain tissue resources have been coordinated on such a scale to study Alzheimer’s disease pathology at every stage of the illness. Dr Johanna Jackson, from our Department of Brain Sciences, said “Developing this resource for all researchers in the field gives us an exciting and unique opportunity to work together to understand the processes involved in Alzheimer’s disease.”
4. New research is examining why people with type 2 diabetes develop dementia
New findings could help identify risk factors for dementia in people with diabetes and inform interventions to help prevent or delay the condition.
Data was analysed from 227,580 people with type 2 diabetes over the age of 42 years, around 10% of whom went on to develop dementia. The team examined the participants’ medical history across the 20 years prior to their dementia diagnosis to look at changes in cardiometabolic factors and bodyweight, and compared these to people who didn’t develop dementia.
 The research, funded by Diabetes UK, analysed ‘cardiometabolic factors’ – such as blood pressure, blood sugars and cholesterol levels and identified changes in these factors that were associated with developing dementia in later life. It has been suggested that these factors that are known to affect heart health might also affect brain health and could potentially play a role in the development of dementia in people with type 2 diabetes.
The research, funded by Diabetes UK, analysed ‘cardiometabolic factors’ – such as blood pressure, blood sugars and cholesterol levels and identified changes in these factors that were associated with developing dementia in later life. It has been suggested that these factors that are known to affect heart health might also affect brain health and could potentially play a role in the development of dementia in people with type 2 diabetes.
Dr Elizabeth Robertson, Director of Research at Diabetes UK, which funded the study, said: “Knowing which factors contribute to the development of dementia, and when they have the biggest impact, is vital in giving people with type 2 diabetes the best possible care to prevent or delay dementia onset.”
5. We’re exploring how genes impact brain cells in Alzheimer’s
New funding of a £1.5 million grant from the Medical Research Council (MRC) will, for the first time, support new research examining changes in how genes function in specific brain cell types to better understand the development of Alzheimer’s disease.
 Previous research has shown that Alzheimer’s disease is characterised by changes occurring in certain cell types, for example, the extensive loss of neurons. Therefore, it is critical to measure gene activity in each different brain cell type individually to understand how they are linked to the development of the condition. Mapping the differences will potentially enable a step-change in unravelling the mechanism of Alzheimer’s disease.
Previous research has shown that Alzheimer’s disease is characterised by changes occurring in certain cell types, for example, the extensive loss of neurons. Therefore, it is critical to measure gene activity in each different brain cell type individually to understand how they are linked to the development of the condition. Mapping the differences will potentially enable a step-change in unravelling the mechanism of Alzheimer’s disease.
Study lead Professor Jonathan Mill, from the University of Exeter, said: “We’re delighted that our project has been funded by the Medical Research Council. By identifying genomic changes in specific cell types in the brain that are associated with Alzheimer’s disease, we will be in a unique position to understand more about the molecular processes involved in this terrible condition and identify pathways that can be hopefully targeted by novel drugs and treatments.”
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Elinor Pegler
Faculty of Engineering