A Versatile Fluorescence Based Assay
Testing has been a fundamental tool in medical diagnosis for many years and increasingly there is a demand for tests that are cost effective and minimally invasive. For example, when it comes to cancer, can we develop a wide range of tests that rely only on small drops of bodily fluids in order to provide information for accurate diagnosis, prognosis and management of the disease? The provision of improved testing in all these areas would provide significant gains over current tests across the board. For example, the diagnosis of prostate cancer is largely reliant upon the PSA test, currently, up to 75% of positive results with the PSA test are false positives and thus there is still a requirement for a follow-up invasive procedure such as a biopsy or digital rectal exam to determine the final diagnosis. In the case of prostate and many other cancers, currently a biopsy is often necessary to determine the prognosis (i.e. what type of cancer, how serious, how aggressive the cancer is). Finally, there is a need to determine the efficacy of a treatment regime being employed. This is especially important in diseases like brain cancer, when a number of treatment options can be available. Monitoring how well a patient responds to a specific treatment can help decide whether this treatment is suitable or whether it needs to be changed.
Biomarkers are measurable indicators of a biological state or condition and new biomarkers in bodily fluids that can assist with diagnostic techniques are being discovered by biologists. However, how to develop reliable, inexpensive and effective ways to measure these biomarkers is a key challenge that has been the focus of the research work of Dr Sylvain Ladame and his team since he joined the College back in 2010.
Nucleic acids form the building blocks of life and have been studied in great detail since DNA molecules were first isolated by Swiss physician Friedrich Miescher in 1869. Later, in 1948, Mandel and Metais discovered that DNA was not just contained within the nuclei of our cells, but fragments of DNA were circulating in the blood stream [1]. More recently, it was proposed that the presence of these cell-free Nucleic Acids (cfNA) in the blood stream can be used to detect specific cancers and it is the problem of how to detect these pieces of DNA junk that has been of particular interest to Sylvain.
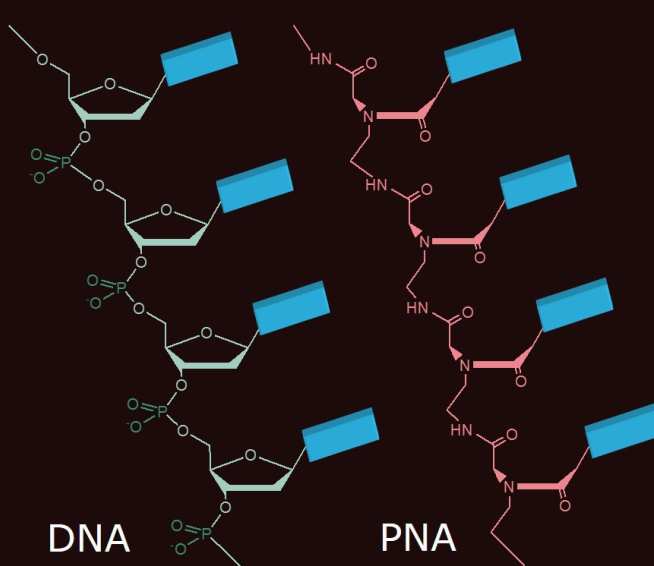
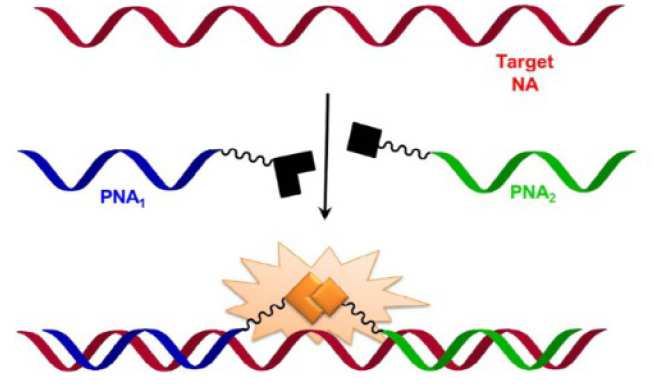
Each piece of DNA is made up of a central scaffold made from sugar and phosphate, onto which are attached nucleobases of four different types, either C, G, A or T. If the sequence of the cfNA biomarkers can be identified, it is possible to construct a corresponding piece of Nucleic Acid that will bind to the floating cfNA and indicate its presence. However, creating these matching strands of either DNA or RNA is difficult and costly, instead Sylvain has been investigating using Peptide Nucleic Acids (PNA) to identify and bind with the floating cfNA. PNAs are artificially synthesised molecules that use peptides instead of sugar and phosphate as the internal structure for the Nucleic Acid, but still having the CGAT bases on the outside. With funding from the EPSRC Impact Acceleration Account, Sylvain has been developing an assay test based upon this principle in which specially designed PNAs fluoresce once bound to a target cfNA to indicate the cfNA’s presence. These PNAs are also relatively inexpensive to produce providing the basis for a low cost non-invasive cancer test which requires only a small drop of a patient’s blood. Initial work for Sylvain has focussed on prostate cancer, however, the technology is potentially applicable to a wide range of cancers. He has also focussed on the platform in which the test could be operated outside the laboratory. For example Sylvain aims to construct a device that could be used in a GPs clinic rather than needing blood samples to be sent off to a specialist laboratory to analyse. Progress has been excellent so far and Sylvain is currently in the process of sourcing funding for the next stage of development that will concentrate on developing the chosen platform for the test.


