Total Synthesis of Bioactive Natural Products
Celastraceae sesquiterpenoids

Crude plant extracts of the Celastraceae genus of plant have been reported to possess: stimulant, appetite suppressive, sedative, emetic, purgative, memory-restorative, anti-fertility, anti-arthritic, anti-tumour, anti-leukemic, anti-bacterial, insecticidal and insect repellent properties! The most notorious plant of the Celastraceae is the Qat or khat plant. This plant is cultivated countries close to the Horn of Africa (e.g. Ethiopia, Kenya, the Yemen Republic) and harvested to be chewed as an apetite suppressant and to combat fatigue.
Many of the isolated compounds from plants of theCelastraceae exhibit potent anti-tumour, insect antifeedant, and anti-HIV activity. However, their extremely low natural abundance and problems with their isolation means that there is an acute supply shortage of these compounds. This precludes exhaustive biological screening and the establishment of meaningful structure activity relationships.
The most pervasive and characteristic secondary metabolites isolated from this class of plants are poly-esters of a family of highly oxygenated sesquiterpenoids having a dihydro-b-agarofuran skeleton (e.g. esters of euonyminol). We are engaged in the total asymmetric synthesis of euonyminol, evoninic acid and iso-evoninic acid which comprise key components of various ANTI-HIV natural products isolated from certain Celastracae plants. We have developed an efficient asymmetric synthesis of a polyoxygenated decalin model system involving a novel modification of the Sharpless asymmetric epoxidation reaction which extends the scope of the reaction to encompass tertiary alchol substrates and we are currently exploring methods for its elaboration to the natural product cores.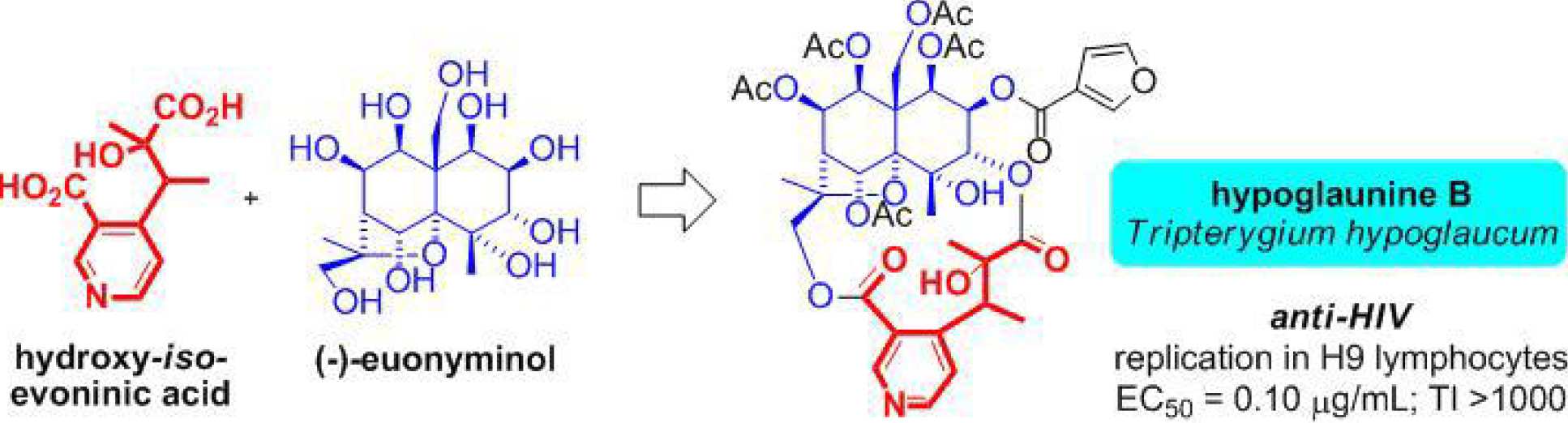
Funded by
-
EPSRC; Hoffmann La Roche, Basle, Switzerland; AstraZeneca Charnwood; Novartis, Horsham
Collaborations with
- none currently.
Selected Publications
- S.A. Warren, S. Stokes, C.S. Frampton, A.J.P. White, A.C. Spivey, 'Synthesis of anti and synHydroxy-iso-Evoninic Acids', Org. Biomol. Chem. 2012, 4685-4688 [PDF(660KB)];
- M.J. Webber, M. Weston, D.M. Grainger, S. Lloyd, S.A. Warren, L. Powell, A. Alanine, J. Stonehouse, C.S. Frampton, A.J.P. White, A.C. Spivey, 'An Ireland–Claisen Rearrangement/Lactonisation Cascade as a Key Step in Studies Towards the Synthesis of (–)-Euonyminol', Synlett 2011, 2693-2696 [PDF (152KB)];
- A.C. Spivey, L. Shukla, J.F. Hayler, 'Conjugate addition of 2- and 4-pyridylcuprates: An expiditious asymmetric synthesis of natural (-)-evoninic acid' Org. Letts. 2007, 9, 891-894. [PDF(184KB)];
- A.C. Spivey, M. Weston, S.J. Woodhead, 'Celastraceae sesquiterpenoids - Biological activity and synthesis' Chem Soc. Rev., 2002, 31, 43-59. [ (2,233KB)];
- A.C. Spivey, S. J. Woodhead, M. Weston, B.I. Andrews, 'Enantioselective desymmetrisation of meso-decalin diallylic alcohols by a new Zr-based Sharpless AE process: a novel approach to the asymmetric synthesis of polyhydroxylated Celastraceae sesquiterpene cores', Angew. Chem. Int. Ed.. 2001, 40, 769-771. [PDF (83KB)]. See also: Publications
Amaryllidaceae alkaloids

The Amarylidaceae genus of plant has a venerable history in relation to the development of organic chemistry as the result of being the source of a wide range of highly bio-active natural products and also because speculation about the biosynthesis of various alkaloids from these plants led to the first postulation of oxidative phenolic coupling as a major mechanism of structural diversity in nature.
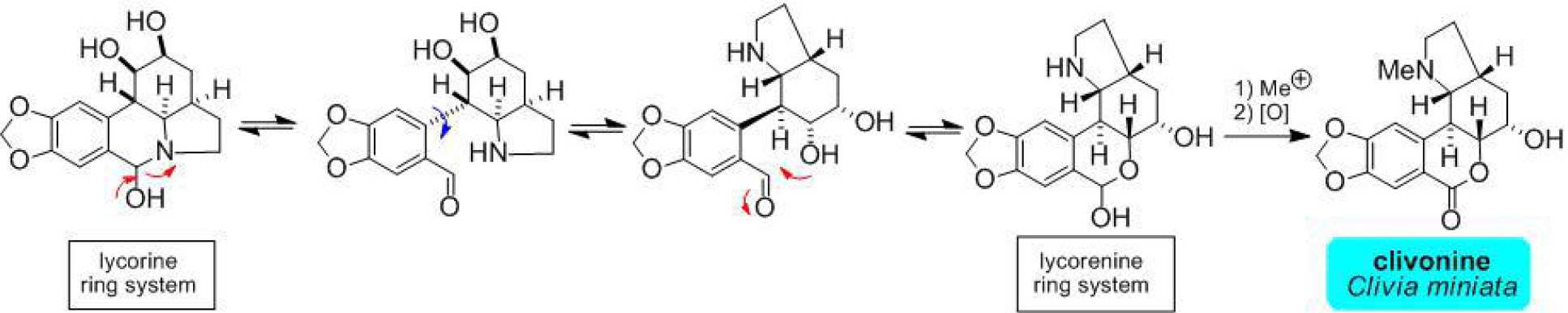
(+)-Clivonine is a lycorenine-class Amaryllidaceae alkaloid which displays insect antifeedant activity. Biosynthetically it is believed to be derived from a lycorine class progenitor by an intramolecular lactamol to lactol exchange equilibrium (involving 180 degree rotation about the C-12a/C-12b bond). We have developed an efficient synthesis of clivonine which exploits a retro-Cope elimination early in the sequence and finishes with a biomimetic interconversion of the lycorine ↔ lycorenine ring systems.
(-)-Lycorine is the most widespread and abundant of the Amaryllidaceae alkaloids. It is a potent emetic, exhibits anti-viral and insect antifeedant activity, inhibits growth of higher plants, algae and yeast by disruption of cell division, inhibits protein and DNA synthesis in murine cells and inhibits in vivo growth of murine transplantable ascite tumours. Our approach to this alkaloid uses a retro-Cope elimination to establish the crucial trans-B-C ring fusion. We are currently working on the final steps in its synthesis.
Funded by
-
EPSRC; GSK, Tonbridge; Erasmus programme
Collaborations with
- none currently
Selected Publications
- H. Haning, C. Giró Mañas, V.L. Paddock, C.G. Bochet, A.J.P. Whit e, G. Bernardinelli, I. Mann, W. Oppolzer, A.C. Spivey 'Total Synthesis of the Amaryllidaceae Alkaloid Clivonine', Org.Biomol. Chem. 2011, 9, 2809-2820 [PDF (558KB)];
- C.G. Manas, V.L. Paddock, C.G. Bochet, A.C. Spivey, A.J.P. White, I. Mann, W. Oppolzer 'Total Synthesis of the Lycorenine-type Amaryllidaceae Alkaloid (±)-Clivonine via a Biomimetic Ring-switch from a Lycorine-type Progenitor', J. Am. Chem. Soc. 2010, 132, 5176-5178. [PDF(645KB)];
- A.C. Spivey, 'Work Hard, Play Hard - a Work Ethic Forged in the Group Oppolzer', Chimia 2009,63, 864-866. [PDF];
- A.C. Spivey, C. Giro Manas, I. Mann, 'Polystyrene-supported N-methylthiourea: a convenient new reagent for the hydrogenolysis of bicyclic endoperoxides' Chem. Commun. 2005, 4426-4428. [PDF (253KB)];
- Bernardinelli, W. Oppolzer, A.C. Spivey, '(2RS)-4,4-Dimethyl-2-[(1SR)-1-Phenethyl]-1-pyrrolidinol', Acta. Cryst. 1995, C51, 529-530. [PDF (523KB)];
- W. Oppolzer, A.C. Spivey, C. Bochet, 'Suprafaciality of thermal N-4-alkenylhydroxylamine cyclizations: syntheses of (±)-alpha-lycorane and (+)-trianthine', J. Am. Chem. Soc., 1994, 116, 3139-3140. [PDF (241KB)]. See also: Publications
Aspergillus sp. fungal metabolites
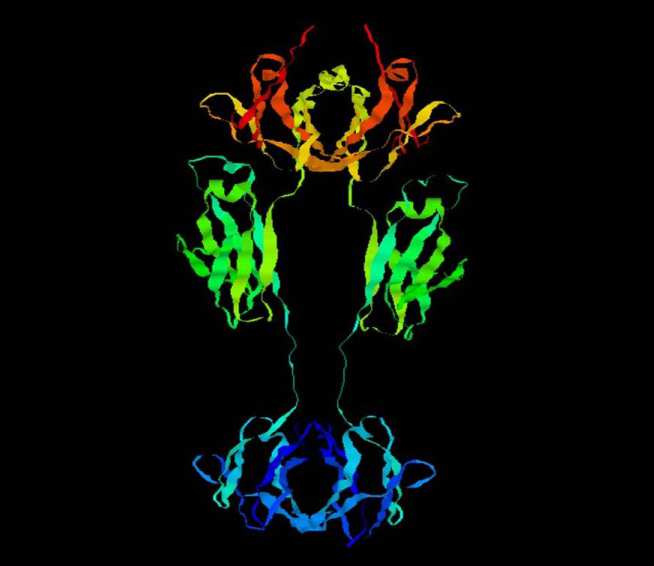
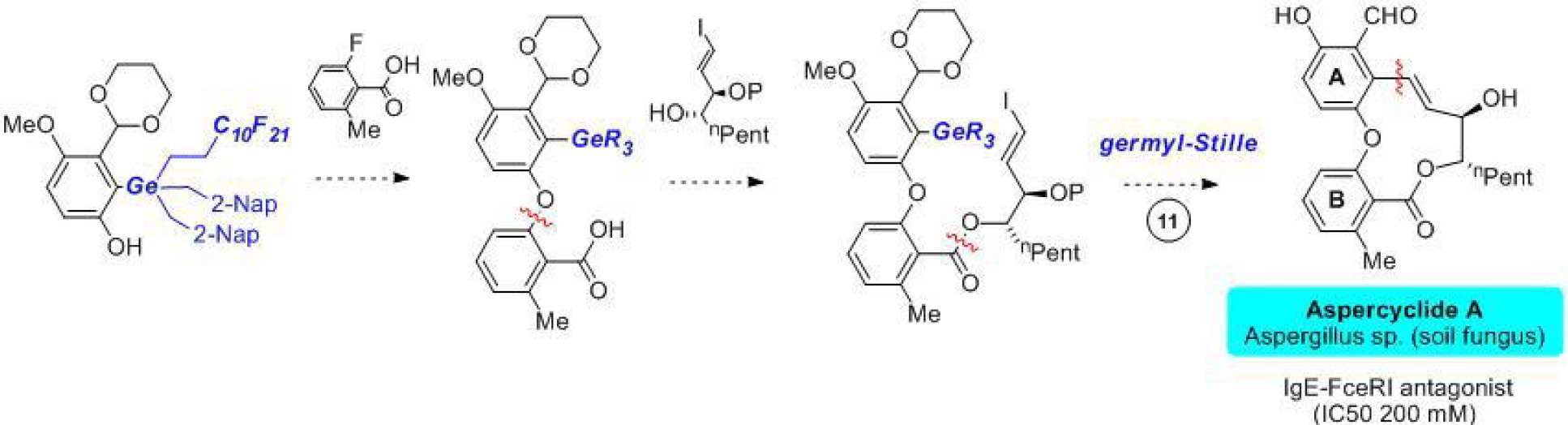
In 2004, Merck reported that a soil-derived fungal metabolite called aspercyclide A was active as an antagonist of the protein-protein interaction (PPI) between human IgE and its high affinity receptor FceRI.
We have completed the synthesis of racemic aspercyclide A methyl ether and are currently working on an asymmetric synthesis of the natural product itself and a series of derivatives to probe SAR for this compound class.
Funded by
-
PSRC; MRC; Bayer CropScience, Frankurt, Germany
Collaborations with
- Prof Brian Sutton for structural biology and protein crystallography of IgE and its receptor (Kings College London, Randall Institute, Guy's Hospital Campus, London, UK). Dr Andrew Beavil for structural and molecular biology of IgE and its receptor (Kings College London, Randall Institute, Guy's Hospital Campus, London, UK).
Selected Publications
- J.L. Carr, J.J.P. Sejberg, F. Saab, M.D. Holdom, A.M. Davies, A.J.P. White, R.J.Leatherbarrow, A.J. Beavil, B.J. Sutton, S.D. Lindell, A.C. Spivey, 'Synthesis of the C19 Methyl Ether of Aspercyclide A via Germyl-Stille Macrocyclisation and ELISA Evaluation of Both Enantiomers Following Optical Resolution', Org. Biomol. Chem. 2011, 9, 6814-6824 [PDF (328KB)];
- J.L. Carr,D.A. Offermann,M.D. Holdom,P. Dusart,A.J.P. White,A.J. Beavil,R.J. Leatherbarrow,S.D. Lindell,B.J. Sutton, A.C. Spivey'Total Synthesis of (+/-)-Aspercyclide A and its C19 Methyl Ether'Chem. Comm. 2010, 1824-1826 [PDF (527KB)]. See also: Publications