"Chemical Photography" of Drug Release
EPSRC project "Application of FTIR Imaging to Drug release" (PI: S. G. Kazarian)
Collaboration with Pfizer, GlaxoSmithKline, Bristol Myers Squibb, Abbott, AbbVie, Unilever and other companies
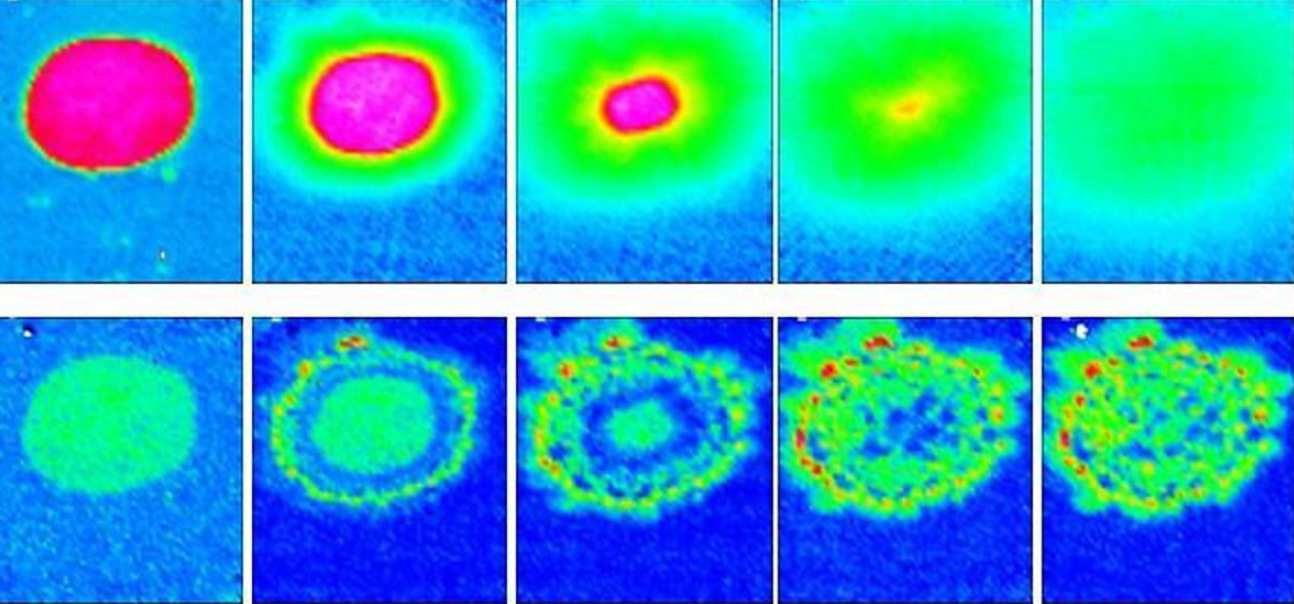 Our group has been active in the development of novel in situ ATR-FTIR spectroscopic imaging approaches for studying of pharmaceutical tablets dissolution and drug release. It is with this approach that we obtained first direct evidence of drug crystallisation during the contact of tablet with water. This observation was surprising since the drug was molecularly dispersed in polymer matrix via supercritical fluid impregnation. The possible re-crystallisation of drug during dissolution was implied in previous studies but our spectroscopic imaging approach has provided the first direct evidence for this phenomenon based on the study of the model drug. One of the main contributions of this work was presenting an ATR-FTIR imaging approach that allowed us to view a dynamic process via simultaneous measurement of the distribution of polymer, drug and water. This spectroscopic imaging method is substantially superior to many of the other imaging methods due to inherent chemical specificity of infrared spectroscopy and fast acquisition times of this technique. Our data demonstrated that the methodology will provide a means to optimise and design formulations for controlled drug delivery, and will help to overcome some of the recognised difficulties in modelling and predicting this phenomenon. Furthermore, this approach is important in a wider technological sense, for example, it will assist studies of water uptake into polymers and adhesives, and the release of active agents in targeted delivery systems such as flavours, fragrances, transdermal drug delivery and personal care products. We also collaborate on the development of near-Infrared (NIR) mode in spectrosocpic imaging for studies of tablet dissolution and drug release (see a separate section of references on NIR below)
Our group has been active in the development of novel in situ ATR-FTIR spectroscopic imaging approaches for studying of pharmaceutical tablets dissolution and drug release. It is with this approach that we obtained first direct evidence of drug crystallisation during the contact of tablet with water. This observation was surprising since the drug was molecularly dispersed in polymer matrix via supercritical fluid impregnation. The possible re-crystallisation of drug during dissolution was implied in previous studies but our spectroscopic imaging approach has provided the first direct evidence for this phenomenon based on the study of the model drug. One of the main contributions of this work was presenting an ATR-FTIR imaging approach that allowed us to view a dynamic process via simultaneous measurement of the distribution of polymer, drug and water. This spectroscopic imaging method is substantially superior to many of the other imaging methods due to inherent chemical specificity of infrared spectroscopy and fast acquisition times of this technique. Our data demonstrated that the methodology will provide a means to optimise and design formulations for controlled drug delivery, and will help to overcome some of the recognised difficulties in modelling and predicting this phenomenon. Furthermore, this approach is important in a wider technological sense, for example, it will assist studies of water uptake into polymers and adhesives, and the release of active agents in targeted delivery systems such as flavours, fragrances, transdermal drug delivery and personal care products. We also collaborate on the development of near-Infrared (NIR) mode in spectrosocpic imaging for studies of tablet dissolution and drug release (see a separate section of references on NIR below)
Key References
- Kazarian S.G., Chan K. L. A. Chemical photography of drug release, Macromolecules 36, (2003), 9866-9872.
- Van der Weerd, J., Chan K. L. A., Kazarian S. G., An Innovative Design of Compaction Cell for In Situ FTIR Imaging of Tablet Dissolution, Vibrational Spectroscopy 35 (2004) 9-13
- Chan K. L. A., Kazarian S. G., FTIR Spectroscopic Imaging of Dissolution of a Solid Dispersion of Nifedipine in Poly(ethylene glycol), Molecular Pharmaceutics 1, (2004) 331-335.
- Kazarian S. G., Kong K. W. T., Bajomo M., van der Weerd J, Chan K. L. A., Spectroscopic imaging applied to drug release, Food and Bioproducts Processing 83(C2) (2005) 127-135.
- Van der Weerd, J., Kazarian S. G. Release of poorly soluble drugs from HPMC tablets studied by FTIR imaging and flow-through dissolution tests, J. Pharmaceutical Sciences. 94 (2005) 2096-2109.
- Kazarian S. G., Van der Weerd J., Simultaneous FTIR spectroscopic imaging and visible photography to monitor tablet dissolution and drug release, Pharmaceutical Research 25, 853-860 (2008).
- Wray, P. S., Clarke, G. S., Kazarian, S. G. Application of FTIR Spectroscopic Imaging to Study the Effects of Modifying the pH Microenvironment on the Dissolution of Ibuprofen from HPMC Matrices Journal of Pharmaceutical Sciences 100(11) (2011) 4745-4755 (doi)
- Wray P. S.; Clarke G.; Kazarian S.G. Dissolution of Tablet-in-Tablet Formulations Studied with ATR-FTIR Spectroscopic Imaging European Journal of Pharmaceutical Sciences (2013) 48 (2013) 748-757 (doi)
- Kazarian S. G., Ewing A. V. Applications of Fourier transform infrared spectroscopic imaging to tablet dissolution and drug release Expert Option on Drug Delivery (2013) 10(9),1207-1221 (doi)
- Pudlas, M., Kyeremateng, S. O., Williams, L. A. M., Kimber, J. A., van Lishaut, H., Kazarian, S. G., Woehrle, G.H.Analyzing the Impact of Different Excipients on Drug Release Behavior in Hot-Melt Extrusion Formulations Using FTIR Spectroscopic Imaging European Journal of Pharmaceutical Sciences (2015) 67, 21–31 (doi)
- Ewing A. V., Wray P.S., Clarke G. S., Kazarian S. G. Evaluating drug delivery with salt formation: drug disproportionation studied in situ by ATR-FTIR imaging and Raman mapping Journal of Pharmaceutical and Biomedical Analysis (2015) 111, 248−256 (doi)
- Hifumi, H., Ewing A. V., Kazarian S. G. ATR-FTIR spectroscopic imaging to study the drying and dissolution of pharmaceutical polymer-based films International Journal of Pharmaceutics (2016) 515, 57–68 (doi) (Open access)
References on applicatins of NIR spectroscopic imaging:
- Ishikawa D., Furukawa D., Tseng, Tsai Wei, Kummetha R.R., Motomura A., Igarashi Y., Sato H., Kazarian S. G., Ozaki Y. High-Speed Monitoring of Crystallinity Change in Poly Lactic Acid during Photodegradable Process by Using a Newly Developed Wide Area NIR Camera (Compovision) Analytical and Bioanalytical Chemistry, (2015) 407(2), 397−403 (doi)
- Ishikawa D., Ishikawa D., ShinzawaH., GenkawaT., Kazarian S. G, Ozaki Y. Recent progress of near-infrared (NIR) imaging – development of novel instruments and their applicability for practical situations Analytical Sciences (2014) 30(1), 143-150 (doi)
- Ishikawa D., Nishii T., Mizano F., Sato H., Kazarian S. G., Ozaki. Y, Potential of a Newly Developed High-Speed Near-Infrared (NIR) Camera (Compovision) in Polymer Industrial Analyses: Monitoring Crystallinity and Crystal Evolution of Polylactic Acid (PLA) and Concentration of PLA in PLA/Poly-(R)-3-Hydroxybutyrate (PHB) Blends Applied Spectroscopy (2013) 67, 1441-1446, (doi)
- Ishikawa D., Nishii T., Mizuno F., Kazarian S. G. Ozaki Y. Development of a high-speed monitoring near infrared hyperspectral camera (Compovision) for wide area imaging and its applications. NIR news24 (2013) 6-10. doi: 10.1255/nirn.1376)
- Ishikawa, D., Murayama, K., Awa, K., Genkawa, T. ; Komiyama, M.; Kazarian, S. G., Ozaki, Y. Application of a Newly Developed Portable NIR Imaging Device to the Dissolution Process Monitoring of Tablets Analytical and Bioanalytical Chemistry (2013) 405, 9401-9409. (doi)