The group is led by Dr Marina Kuimova and is based in the Chemistry Department at Imperial College London. In addition, the group is associated with the Institute of Chemical Biology.
Our current work uses variety of imaging and spectroscopic techniques to elucidate the nature of processes involved in cell function and death, including those during Photodynamic Therapy (PDT) treatment of cancer. My research interests also include ultrafast processes involving excited states of biomolecules, such as DNA, and photophysics of coordination compounds.
Current research themes
- Molecular rotors
- Imaging DNA G-quadruplexes (G4s)
- Imaging atmospheric aerosols
- Two-Photon Photodynamic Therapy (TPE PDT)
- Measuring intracellular singlet oxygen
Molecular rotors are small fluorophores in which fluorescence competes with intramolecular rotation. In a viscous environment rotation is slowed down and this strongly affects fluorescence parameters, such as intensity, decay time and spectral profile. We have designed rotors which allow to measure viscosity by changes in their fluorescence spectra or lifetimes. We have studied the effect of temperature and polarity on molecular rotors and used them in a variety of applications.
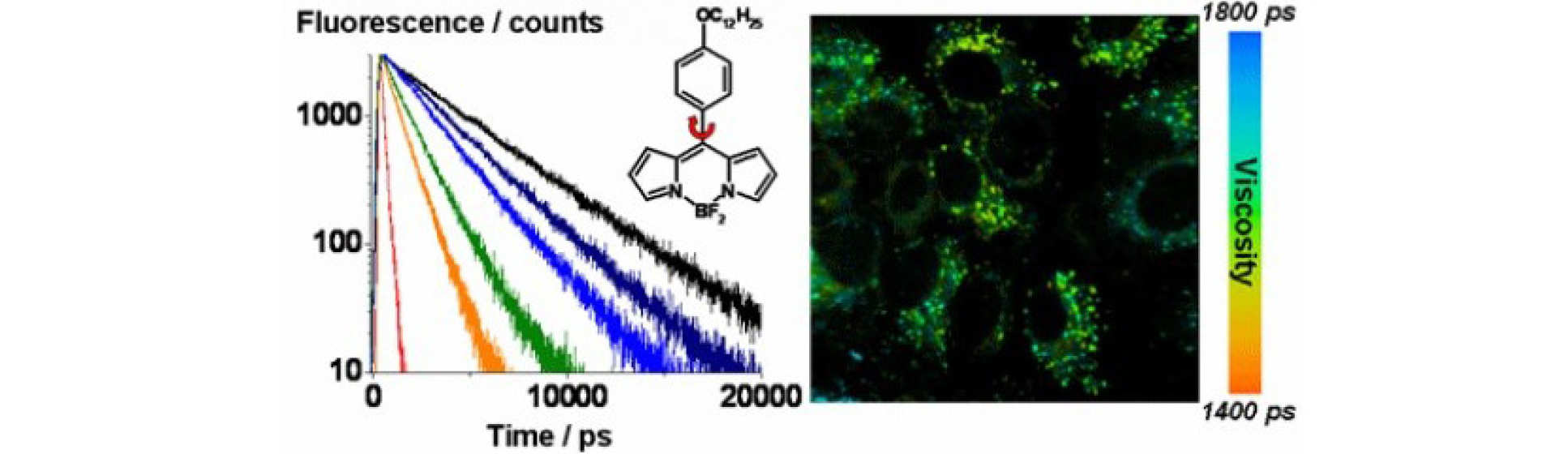
A. Vysniauskas and M. K. Kuimova, A twisted tale: measuring viscosity and temperature of microenvironments using molecular rotors, International Reviews Phys Chem, 2018
M. K. Kuimova, G. Yahioglu, J. A. Levitt, K. Suhling, Molecular Rotor Measures Viscosity of Live Cells via Fluorescence Lifetime Imaging, J. Amer. Chem. Soc., 2008, 130, 6672
M. K. Kuimova, S. W. Botchway, A. W. Parker, M. Balaz, H. A. Collins, H. L. Anderson, K. Suhling, P. R. Ogilby, Imaging Intracellular Viscosity of a Single Cell During Photoinduced Cell Death, Nature Chem., 2009, 1, 69
Guanine rich oligonucleotides can fold into secondary structures known as G-quadruplexes, which are proposed to have various biological roles, however, their visualisation in live cells still remains a challenge. We are using Fluorescence Lifetime Imaging microscopy to quantitatively and dynamically image the presence of G4s inside live cells.
Our recently discovered cell-permeable, low-toxicity sensor enabled the quantification and dynamic monitoring of this rare DNA topology in live cells for the first time. This work is in close collaboration with the groups of Professor Ramon Vilar and Jean Baptiste Vannier.
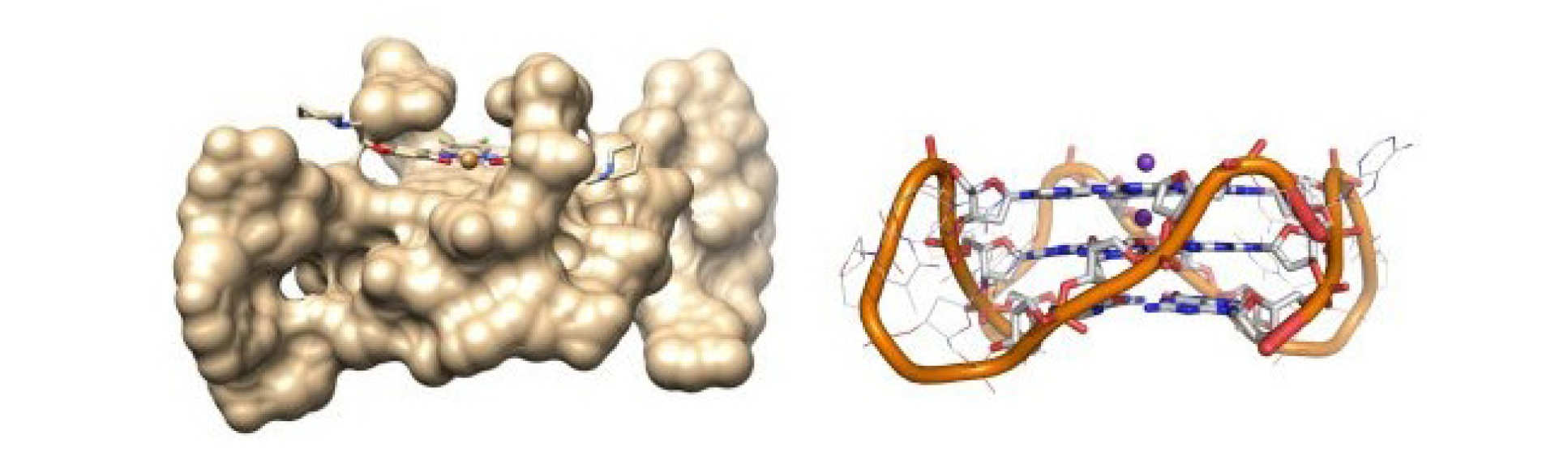
A. Shivalingam et al, The interactions between a small molecule and G-quadruplexes are visualized by fluorescence lifetime imaging microscopy, Nature Communications volume 6, Article number: 8178 (2015)
Organic aerosol particles play major roles in atmospheric chemistry, climate, and public health. There is recent evidence that they might be present in highly viscous states, however, direct observational evidence is lacking, as are methods that are able to dynamically quantify and track viscosity changes during atmospherically relevant processes. We are using molecular rotors to provide quantitative real-time, online observation of aerosol microscopic viscosity in atmospherically relevant aerosol particles.Such data cannot be obtained with any other currently used technique. We have designed and characterised molecular rotors suitable for the particularly high viscosity ranges needed for aerosol mapping.
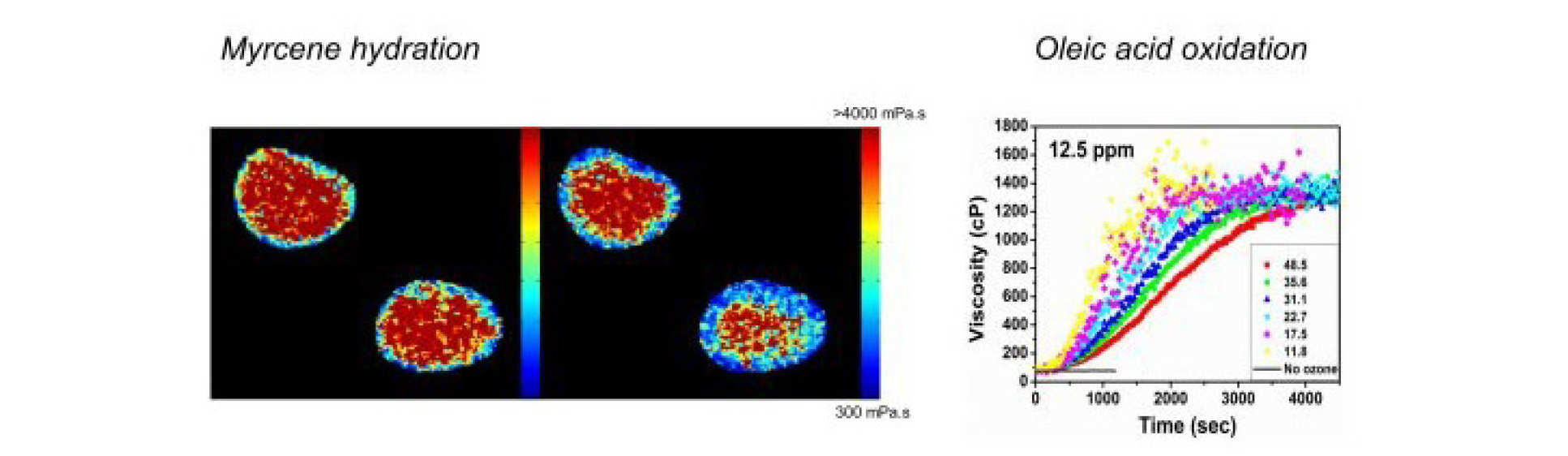
N. A. Hosny et al, 'Direct imaging of changes in aerosol particle viscosity upon hydration and chemical aging' Chemical Science 2015.
A. Athanasiadis et al, Dynamic viscosity mapping of the oxidation of squalene aerosol particles, PCCP, 2016
C. Fitzgerald, Fluorescence lifetime imaging of optically levitated aerosol: a technique to quantitatively map the viscosity of suspended aerosol particles, PCCP 2016
PDT is a clinically tested promising technique to treat cancer. PDT uses red light to activate light-sensitive drugs (photosensitizers) to produce short lived cytotoxic species such as singlet oxygen to destroy malignant cells. We investigate mechanisms of cell death during PDT using fluorescence microscopy and imaging and perform spectroscopic measurements to help design better photosensitizers.
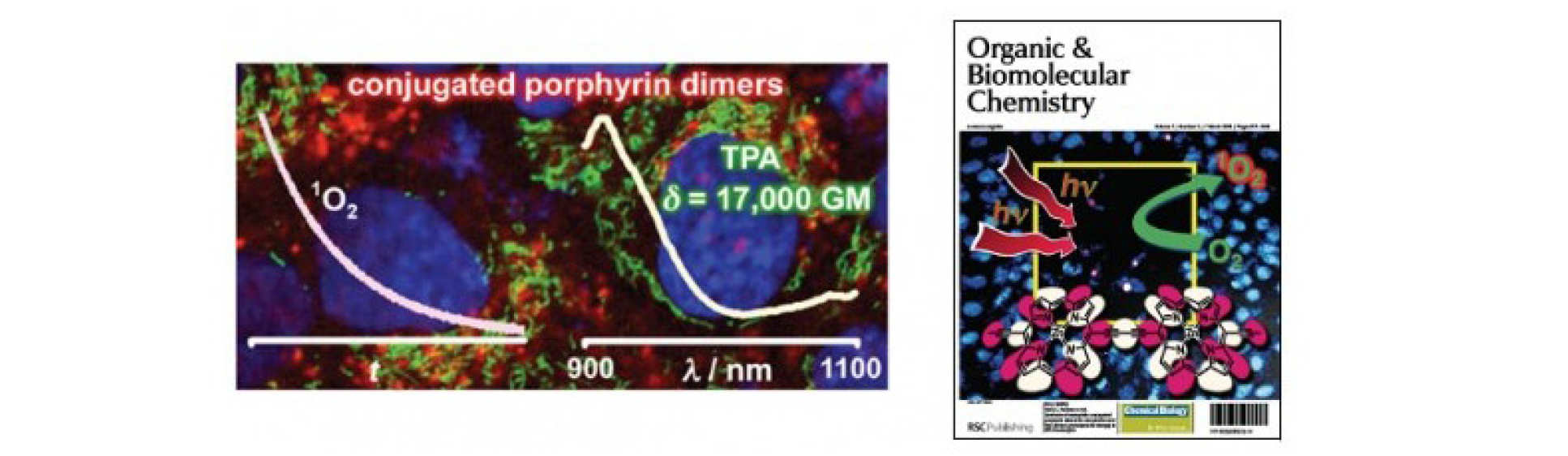
M. K. Kuimova, H. A. Collins, M. Balaz, E. Dahlstedt, J. A. Levitt, N. Sergent, K. Suhling, M. Drobizhev, N. S. Makarov, A. Rebane, H. L. Anderson and D. Phillips, Photophysical properties and intracellular imaging of water-soluble porphyrin dimers for two-photon excited photodynamic therapy, Org. Biomol. Chem., 2009, 7, 889
M. K. Kuimova, M. Bhatti, M. Deonarain, G Yahioglu, J. A. Levitt, I. Stamati, K. Suhling, D. Phillips, Fluorescence characterisation of multiply-loaded anti-HER2 single chain Fv-photosesitizer conjugates suitable for photodynamic therapy, Photochem. Photobiol. Sci., 2007, 6, 933
M. K. Kuimova, M. Balaz, H. L. Anderson, P. R. Ogilby, Intramolecular rotation in a porphyrin dimer controls singlet oxygen production, J. Amer. Chem. S oc., 2009, 131 (23), 7948
Singlet oxygen is the main cytotoxic agent in PDT. Since the lifetime and reactivity of this species is d etermined to a large extent by diffusion, monitoring singlet oxygen in a cell provides us with a sensitive probe of viscosity.
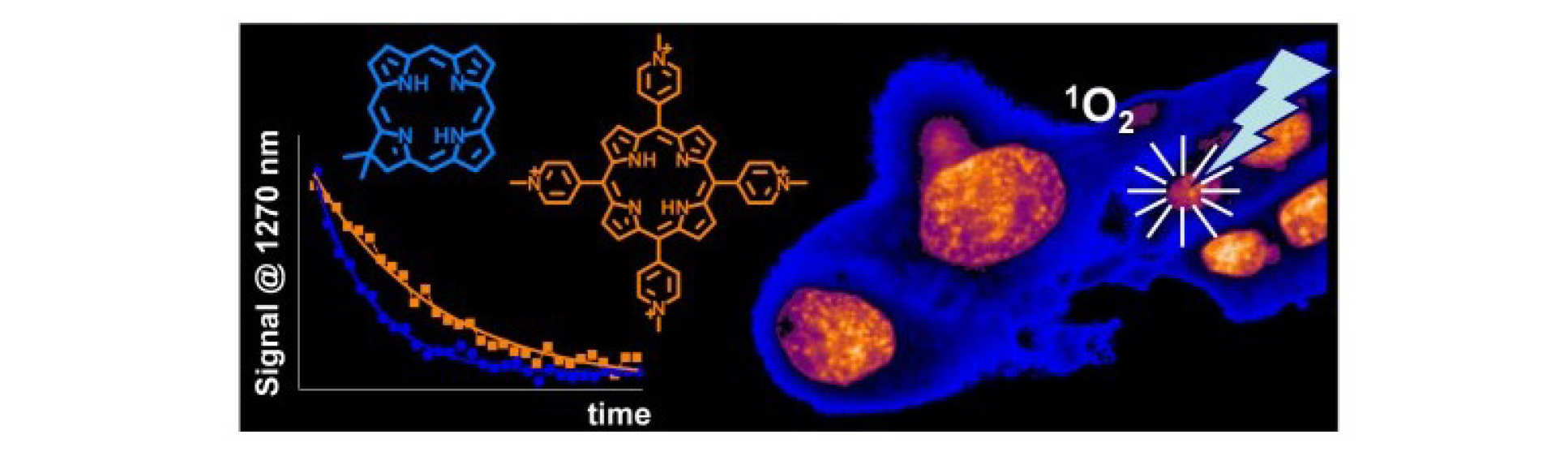
M. K. Kuimova, G. Yahioglu and P. R. Ogilby, Singlet Oxygen in a Cell: Spatially-Dependent Lifetimes and Quenching Rate Constants, J. Amer. Chem. Soc., 2009, 131 (1), 332
M. K. Kuimova, S. W. Botchway, A. W. Parker, M. Balaz, H. A. Collins, H. L. Anderson, K. Suhling, P. R. Ogilby, Imaging Intracellular Viscosity of a Single Cell During Photoinduced Cell Death, Nature Chem., 2009, 1, 69