The research interests of the group encompass a broad range of topics including synthetic coordination and organometallic chemistry as well as materials science. Applications being addressed through this research include catalysis, medical imaging, sensing and platform chemicals from renewable sources.
Some current areas of investigation are outlined below:
Research
- Sensing of carbon monoxide and other analytes
- Molecular and nanoparticle-based imaging and therapy
- Recovery and re-use of metals in catalysis
- Breakdown and conversion of biomass using ILs
- Functionalised nanostructures
- Multimetallic complexes
- NHC-derived dithiocarboxylate ligands

In collaboration with the group of Prof. Ramon Martinez-Manez (UP Valencia, Spain), we have used transition metal complexes to sense low levels of carbon monoxide in the solids state. This can be achieved through a dramatic colour change as well as an increase in fluorescence (JACS 2014). Importantly, the complexes are designed to be selective for carbon monoxide over other species which may be present such as water, carbon dioxide etc. The application of this approach to the sensing of endogenous CO produced in vivo as an marker of inflammation has also been achieved (JACS 2017, Phys.org news article). More recently, the probe design has been adapted to allow simultaneous measurement of the presence of CO and the local viscosity in the cellular environment (Angew. Chem. Int. Ed. 2020). This collaboration with Prof. Marina Kuimova makes use of a combination of fluorescence and fluorescence lifetime imaging microscopy (FLIM) and is the first example of CO and viscosity changes, which are both markers of disease, being measured by the same probe. This research has featured on the College website and in Chemistry & Engineering News.
M. E. Moragues, A. Toscani, F. Sancenon, R. Martinez-Manez, A. J. P. White, J. D. E. T. Wilton-Ely, J. Am. Chem. Soc. 2014, 136, 11930 [DOI: 10.1021/ja507014a].
A. Toscani, C. Marin-Hernandez, M. E. Moragues, F. Sancenon, P. Dingwall, N. J. Brown, R. Martinez-Manez, A. J. P. White, J. D. E. T. Wilton-Ely, Chem. Eur. J. 2015, 21, 14529 [DOI: 10.1002/chem.201501843].
A. Toscani, C. Marín-Hernández, F. Sancenón, R. Martínez-Máñez, J. D. E. T. Wilton-Ely, Chem. Commun., 2016, 52, 5902 (cover article) [DOI: 10.1039/C6CC01335J].
C. de la Torre, A. Toscani, C. Marín-Hernández, J. A . Robson, M. C. Terencio, A. J. P. White, M. J. Alcaraz, J. D. E. T. Wilton-Ely, R. Martínez-Máñez, F. Sancenón, J. Am. Chem. Soc. 2017, 139, 18484 [DOI: 10.1021/jacs.7b11158].
A. Toscani, C. Marín-Hernández, J. A . Robson, E. Chua, P. Dingwall, A. J. P. White, F. Sancenón, C. de la Torre, R. Martínez-Máñez, J. D. E. T. Wilton-Ely, Chem. Eur. J. 2019, 25, 2069 [DOI: 10.1002/chem.201805244].
J. García-Calvo, J. A. Robson, T. Torroba, J. D. E. T. Wilton-Ely, Chem. Eur. J. 2019, 25, 14214 [DOI: 10.1002/chem.201903303] [Hot Paper]
J. A. Robson, M. Kubánková, T. Bond, R. A. Hendley, A. J. P. White, M. K. Kuimova, J. D. E. T. Wilton-Ely, Angew. Chem. Int Ed. 2020, 59, 21431–21435 [DOI: 10.1002/anie.202008224].
Although the majority of our work on sensing is focused on carbon monoxide, whether in air or in biological environments, the group also investigates other analytes. For example, the presence of heavy metals in aqueous solution is of enormous concern and so their detection at very low levels is of great importance. We collaborate with Prof. Joshua Edel and Prof. Alexei Kornyshev (both Imperial Chemistry) to achieve the selective detection of mercury ions at very low concentrations using functionalised gold nanoparticles and Surface Enhanced Raman Spectroscopy (see diagram below).
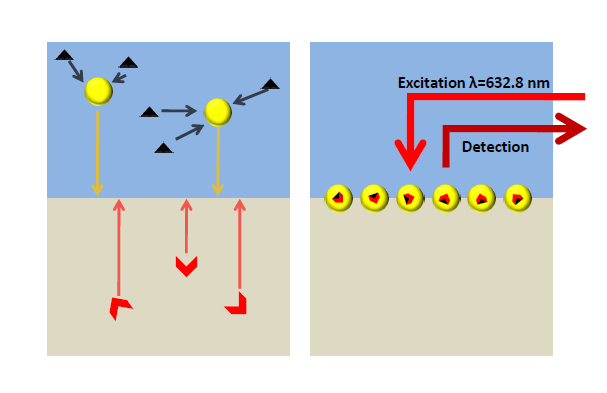
M. P. Cecchini, V. A. Turek, A. Demetriadou, G. J. Britovsek, T. Welton, A. Kornyshev, J. D. E. T. Wilton-Ely, J. B. Edel, Adv. Optical Mater. 2014, 2, 966 (cover article) [DOI: 10.1002/adom.201400211].
Multifunctional MRI contrast agents
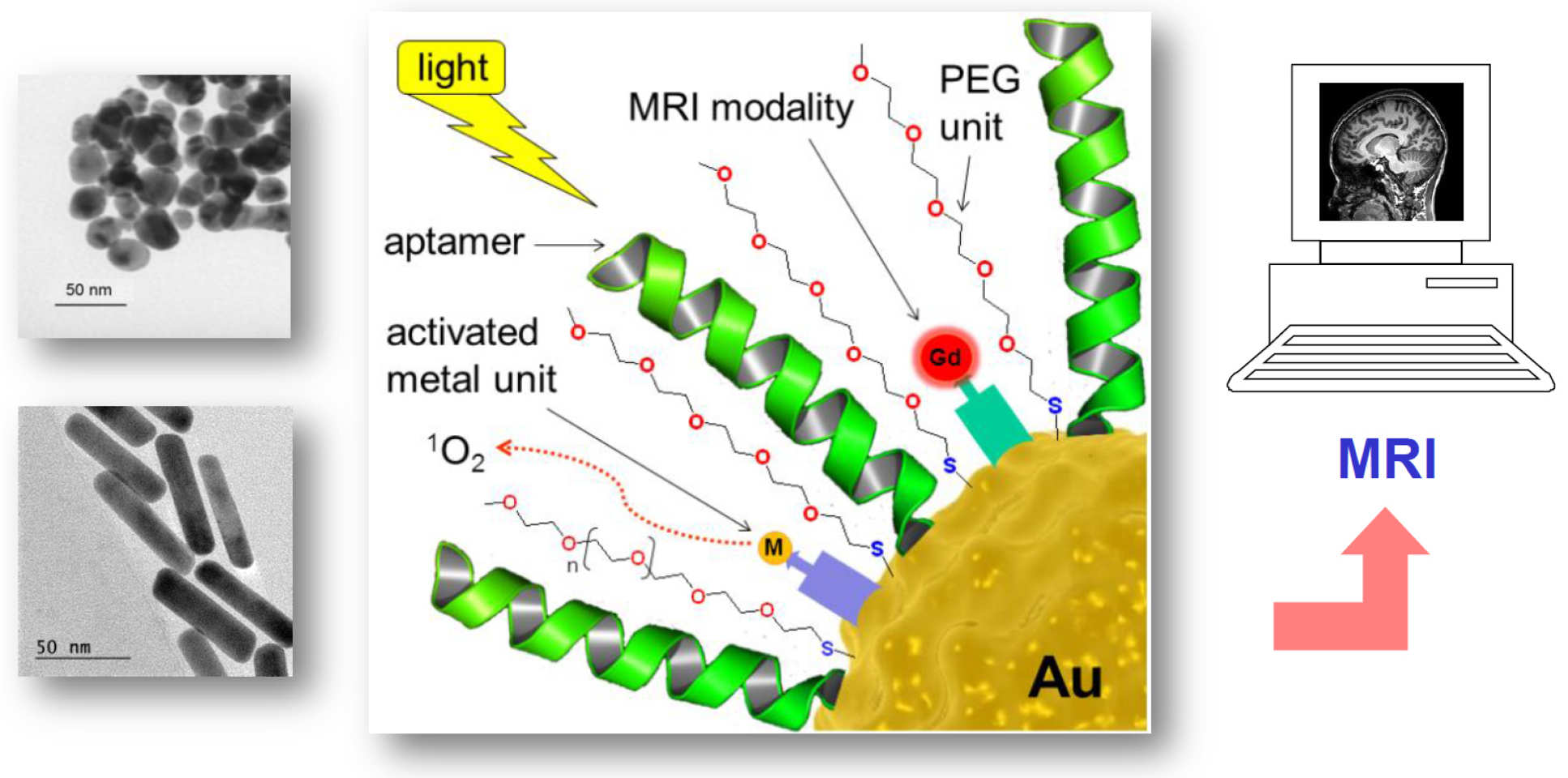
In collaboration with Prof. Rene Botnar (St. Thomas' Hospital, KCL) and Dr Graeme Stasiuk (KCL), we are designing new magnetic resonance imaging (MRI) contrast agents. MRI is a powerful, non invasive technique used to diagnose disease in patients. Improvement to the contrast of the images is achieved using paramagnetic (but toxic) gadolinium(III) ions. Two approaches using Gd(III) are being employed to develop more efficient and targeted contrast agents. The first involves the construction of assemblies with three Gd(III) ions connected to a central transition metal node, which can be also be used to introduce a second imaging modality (optical, PET) into the system (Inorg. Chem. 2020). An alternative approach, based on the same methodology, is the functionalisation of gold nanoparticles with Gd(III) units (Chem. Eur. J. 2019 and 2020). The control over the size and surface units of these nanoparticles allow greater targeting of the agent, potentially allowing lower doses to be employed. We are currently working with Prof. Dan Elson (Imperial, Department of Surgery and Cancer) to add a photo-switchable therapeutic action to these materials, through the use of gold nanorods (Nanotheranostics 2021).
 See also: KCL-IC Centre for Doctoral Training in Smart Medical Imaging and MRC Clinical Sciences Centre
See also: KCL-IC Centre for Doctoral Training in Smart Medical Imaging and MRC Clinical Sciences Centre
S. Sung, H. Holmes, L. Wainwright, A. Toscani, G. J. Stasiuk, A. J. P. White, J. D. Bell, J. D. E. T. Wilton-Ely, Inorg. Chem. 2014, 53, 1989 [DOI: 10.1021/ic401936w].
M. Rasekh, Z. Ahmad, R. Cross, J. Hernandez-Gil, J. D. E. T. Wilton-Ely, P. W. Miller, Mol. Pharmaceutics 2017, 14, 2010-2023 [DOI: 10.1021/acs.molpharmaceut.7b00109].
N. G. Chabloz, M. N. Wenzel, H. L. Perry, I.-C. Yoon, S. Molisso, G. J. Stasiuk, D. S. Elson, A. E. G. Cass, James D. E. T. Wilton-Ely, Chem. Eur. J. 2019, 25, 10895–10906 [doi: 10.1002/chem.201901820] [Hot Paper]
N. G.Chabloz, H. L. Perry, I.-C. Yoon, A. J. Coulson, A. J. P. White, G. J. Stasiuk, R. M. Botnar, J. D. E. T. Wilton-Ely, Chem. Eur. J. 2020, 26, 4552–4566 [DOI: 10.1002/chem.201904757]
H. L. Perry, I.-C. Yoon, N. G. Chabloz, S. Molisso, G. J. Stasiuk, R. M. Botnar, J. D. E. T. Wilton-Ely, Inorg. Chem. 2020, 59, 10813–10823 [DOI: 10.1021/acs.inorgchem.0c01318]
H. L. Perry, R. M. Botnar and J. D. E. T. Wilton-Ely, Chem. Commun. 2020, 56, 4037–4046 [invited review] [DOI: 10.1039/D0CC00196A]
A. Guinart, H. L. Perry, J. D. E. T. Wilton-Ely, T. D. Tetley, Emerging Top. Life Sci. 2020, 4, 627–643 [DOI: 10.1042/ETLS20200332]
X. Shi, H. L. Perry, J. D. E. T. Wilton-Ely, Nanotheranostics 2021, 5, 155-165 [DOI: 10.7150/ntno.56432]
Imaging and sensing using dual modality fluorescent PET imaging probes
Positron Emission Tomography (PET) is a powerful technique, used particularly in oncology, which allows three-dimensional imaging of tissue deep in the body (2 million scans in the US each year). However, substantial infrastructure is required for (often short-lived) radioisotope generation. Together with colleagues at St. Thomas' Hospital (KCL), we are working to incorporate fluorescence within the same agent in order to allow imaging through the emission of visible light to indicate the location of the agent. Adding targeting units to the probe ensures high selectivity for tumours, thus creating a targeted, dual modality agent for the imaging of cancer. Importantly, this will allow visualisation of the tumour site before an invasive procedure (using PET) and, once radiation is no longer present, during surgery (using the fluorescence).
It has only recently been established that our bodies naturally produce and use carbon monoxide as a gaseous messenger. Using a fluorescence response, similar probes to those above are being investigated for the real-time monitoring of carbon monoxide in this role. Based on related systems we have developed (J. Am. Chem. Soc. 2014, 136, 11930, J. Am. Chem. Soc., 2017, 139, 18484−18487), such systems could solve the problems of low sensitivity and response speed which undermine current approaches in this new field. In addition, the presence of abnormal levels of endogenous carbon monoxide has been shown to be a marker of disease, providing clinical relevance. 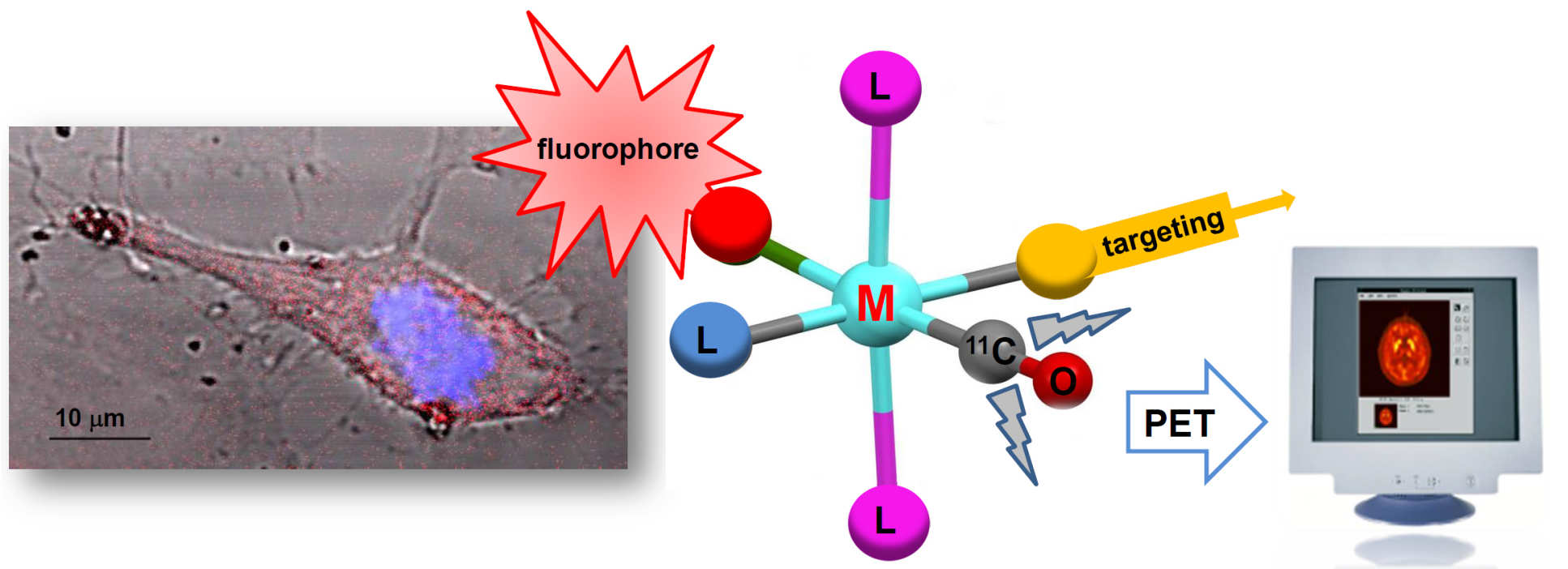 See also: KCL-IC Centre for Doctoral Training in Smart Medical Imaging and MRC Clinical Sciences Centre
See also: KCL-IC Centre for Doctoral Training in Smart Medical Imaging and MRC Clinical Sciences Centre
Precious metals such as Rh, Pd, Pt, Au underpin a vast range of technological processes. They are obtained using often environmentally-damaging mining processes, which have substantial impact on the local communities. The existing deposits are located in particular countries and their supply and price can often be compromised by geopolitical events. These factors have placed increasing pressure on the traditional sources of precious metals. One way to counter this is to recycle and re-use these metals from secondary sources such as automotive scrap and electrical and electronic waste (WEEE).
We collaborate with Professor Angela Serpe at the University of Cagliari in Sardinia, Italy, who is an expert on recovering metals from waste using mild often sulfur-based ligands. Together with students from the MRes in Green Chemistry and MRes in Catalysis courses, we are working on applying directly in catalysis the molecular metal complexes produced by the patented process pioneered by Professor Serpe, which is now in the pilot plant stage.
We have shown (Green Chem. 2017; ACS Sustainable Chem. Eng. 2019) how molecular palladium complexes can be recovered selectively from used automotive catalytic converters and be directly valorised as highly-active catalysts for C-H oxidative functionalisation reactions (Imperial College article). This avoids the high energy cost of passing through palladium metal or its salts, thus creating homogenous catalysts from used heterogeneous catalysts. This approach combines many fundamental concepts of green chemistry through the use of safe reagents and mild conditions to recover and re-use metals for use in a catalytic transformation. This fits very well to the ‘circular economy’ concept, which seeks the best means of valorising the output of a recovery process. This and other complementary approaches are at the forefront of efforts to make palladium catalysis more sustainable (Coord. Chem. Rev. 2021).
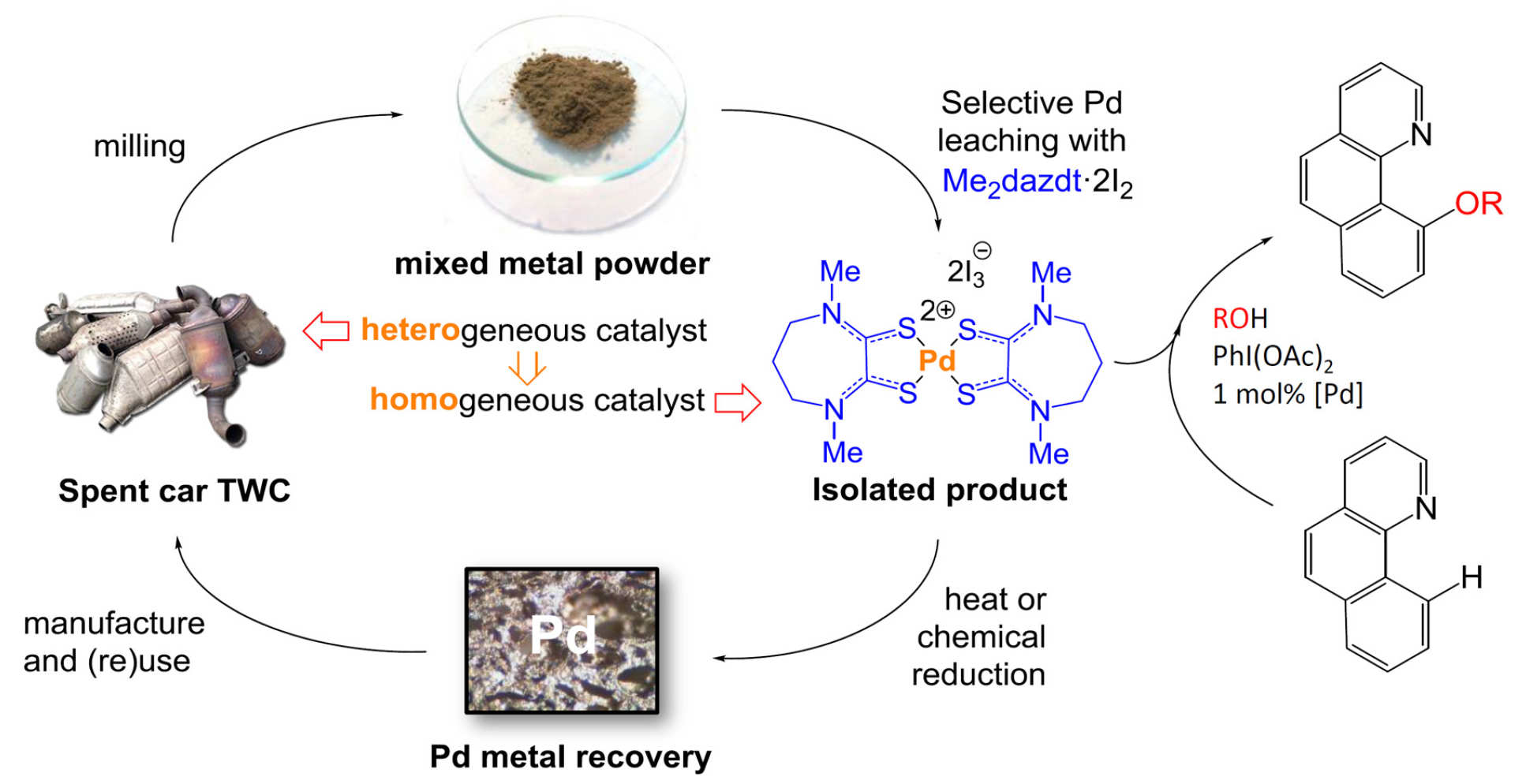
K. A. Jantan, C. Y. Kwok, K. W. Chan, L. Marchio, A. J. P. White, P. Deplano, A. Serpe, J. D. E. T. Wilton-Ely, Green Chem. 2017, 19, 5846-5853 [DOI: 10.1039/C7GC02678A].
K. A. Jantan, K. W. Chan, L. Melis, L. Marchio, A. J. P. White, P. Deplano, A. Serpe, J. D. E. T. Wilton-Ely, ACS Sustainable Chem. Eng. 2019, 7, 12389−12398 [DOI: 10.1021/acssuschemeng.9b01877].
S. McCarthy, D. C. Braddock, J. D. E. T. Wilton-Ely, Coord. Chem. Rev. 2021, 442, 213925 [DOI: 10.1016/j.ccr.2021.213925].
S. McCarthy, A. Lee Wei Jie, D. C. Braddock, A. Serpe, J. D. E. T. Wilton-Ely, Molecules 2021, 26, 5217 [DOI: 10.3390/molecules26175217].
Together with Professor Chris Braddock (Imperial Chemistry) and Prof. Serpe, we have demonstrated very recently that a gold recovery product obtained from real e-waste, such as SIM cards, can be used as an effective homogeneous catalyst for a range of catalytic reactions. This has resulted in an existing mild and effective recovery process to be connected with a major catalytic application of gold. The [AuI2(Me2dazdt)]+ recovery product was found to show comparable catalytic activity to current Au(III) catalysts for a range of important gold-catalysed transformations, such as those shown below.
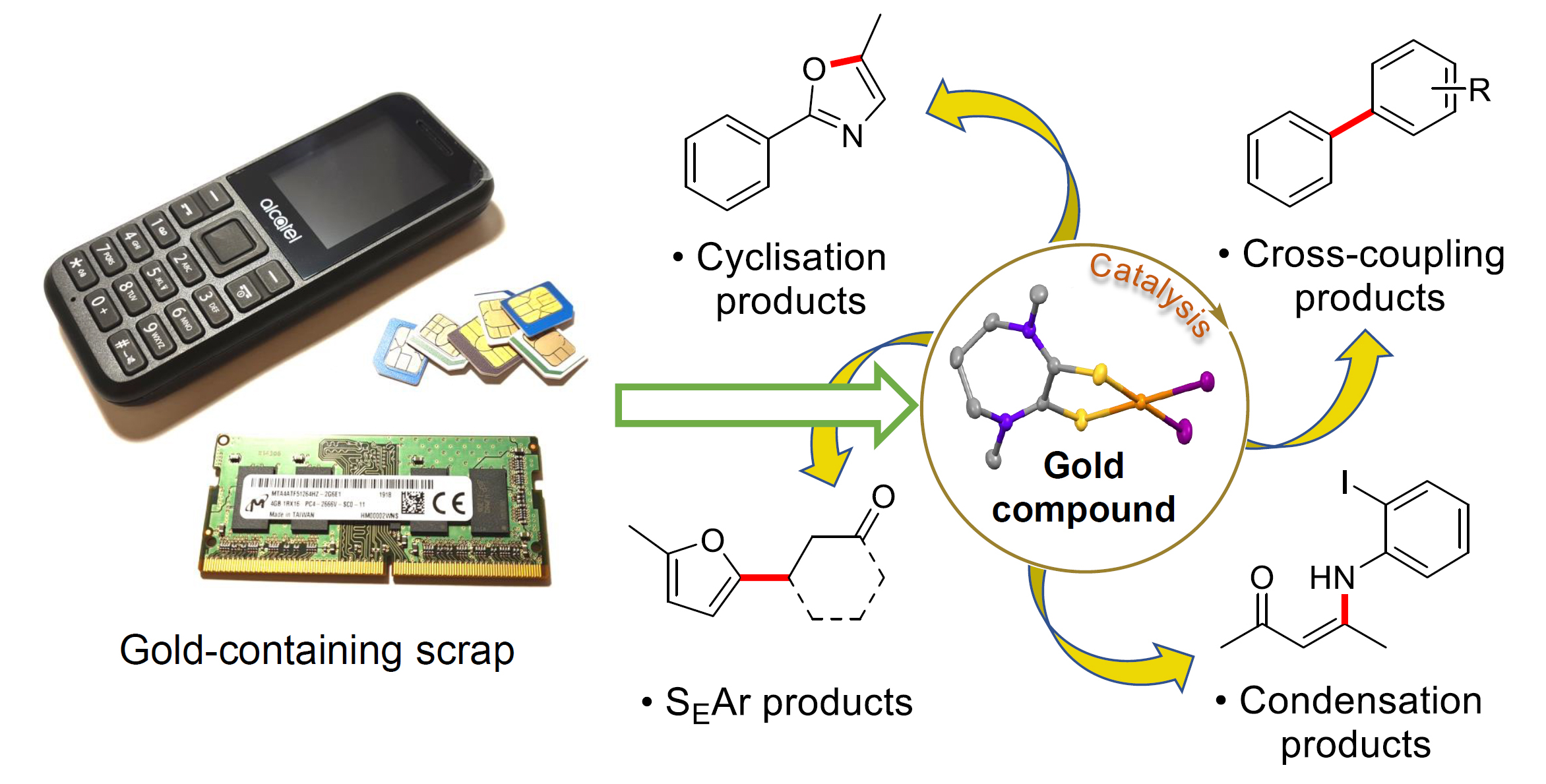
This was the first example of the direct application in homogeneous catalysis of gold recovery products sourced from e-waste. A preliminary indicative cost analysis suggests that even unoptimized, small-scale production of the recovered gold catalyst leads to a significantly lower cost than commercial catalysts derived from environmentally-damaging mining.
This work featured on the College website and received widespread attention in the media, leading to articles in Popular Science magazine, the Times of India and Al Jazeera [in Arabic].
The wider aim of these studies is to demonstrate that molecular recovery products from mild, low-impact processes based on end-of-life materials can be used to replace homogeneous catalysts derived from mining. If adopted more widely, this would not only improve sustainability, reduce waste and relieve pressure on primary sources, but also have cost and supply chain security benefits.
S. McCarthy, O. Desaunay, A. Lee Wei Jie, M. Hassatzky, A. J. P. White, P. Deplano, D. C. Braddock, A. Serpe, J. D. E. T. Wilton-Ely, ACS Sustainable Chem. Eng. 2022, 10, 15726 [DOI: 10.1021/acssuschemeng.2c04092]
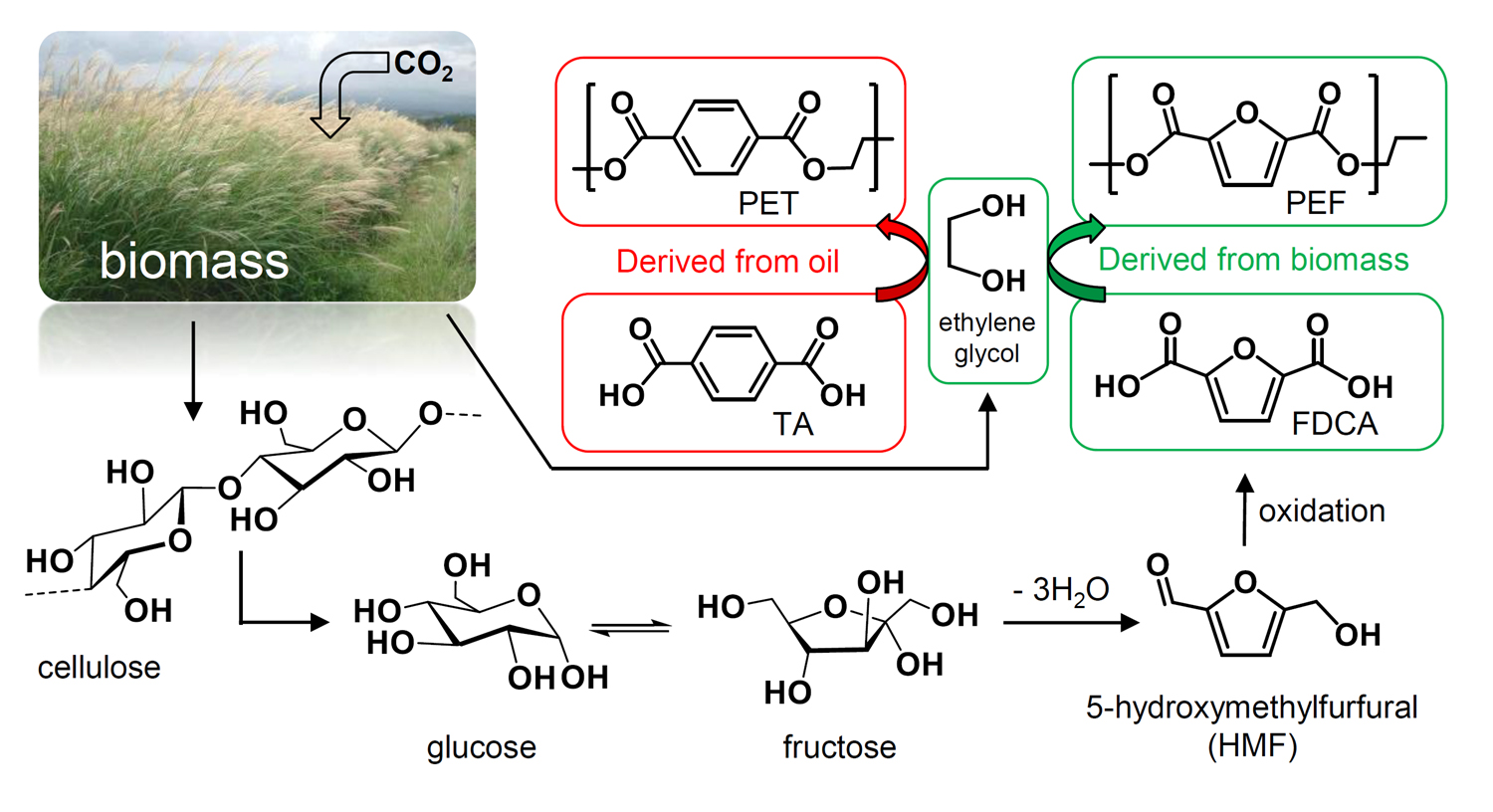
In collaboration with Prof. Jason Hallett, (Imperial Chemical Engineering), we have investigated the dissolution and breakdown of woody biomass using transition metal catalysts in ionic liquids. Ionic liquids are solvents which can be tuned to possess specific properties and they are exceptionally good at dissolving the cellulosic component of biomass. We have used them to develop a process to dissolve refined biomass (fructose, glucose and cellulose) and convert it to 5-hydroxymethylfurfural (HMF) in high yield using low catalyst loadings. HMF has been identified as a key platform chemical which could be used as an alternative to petroleum-sourced building blocks in the future. The transition from a traditional oil-based refinery to a biorefinery, which uses biomass as its feedstock, is likely to use HMF as a key platform chemical product and so it is imperative to develop methods for its efficient production. Further transformations into other useful building blocks such as monomers for polymerisation are also under way.
S. Eminov, J. D. E. T. Wilton-Ely, J. P. Hallett, ACS Sustainable Chem. Eng. 2014, 2, 978 [DOI: 10.1021/sc400553q].
S. Eminov, A. Brandt, J. D. E. T. Wilton-Ely, J. P. Hallett, PLoS One 2016, 11, e0163835 [DOI: 10.1371/journal.pone.0163835].
S. Eminov, P. Filippousi, A. Brandt, J. D. E. T. Wilton-Ely, J. P. Hallett, Inorganics 2016, 4, 32 [DOI: 10.3390/inorganics4040032] .
A. Al Ghatta, J. D. E. T. Wilton-Ely and J. P. Hallett, ChemSusChem 2019, 12, 4452–4460 [DOI: 10.1002/cssc.201901529].
Due to the high boiling point and instability of HMF and the high energy costs and organic solvents required in isolating it, the potential for converting HMF to the versatile platform chemical 2,5-diformylfuran (DFF) was explored. It was found that this could be achieved using an immobilised TEMPO system with a copper co-catalyst at only 1 atm oxygen pressure. Isolation of the DFF product in high purity was achieved through sublimation from the ionic liquid medium.
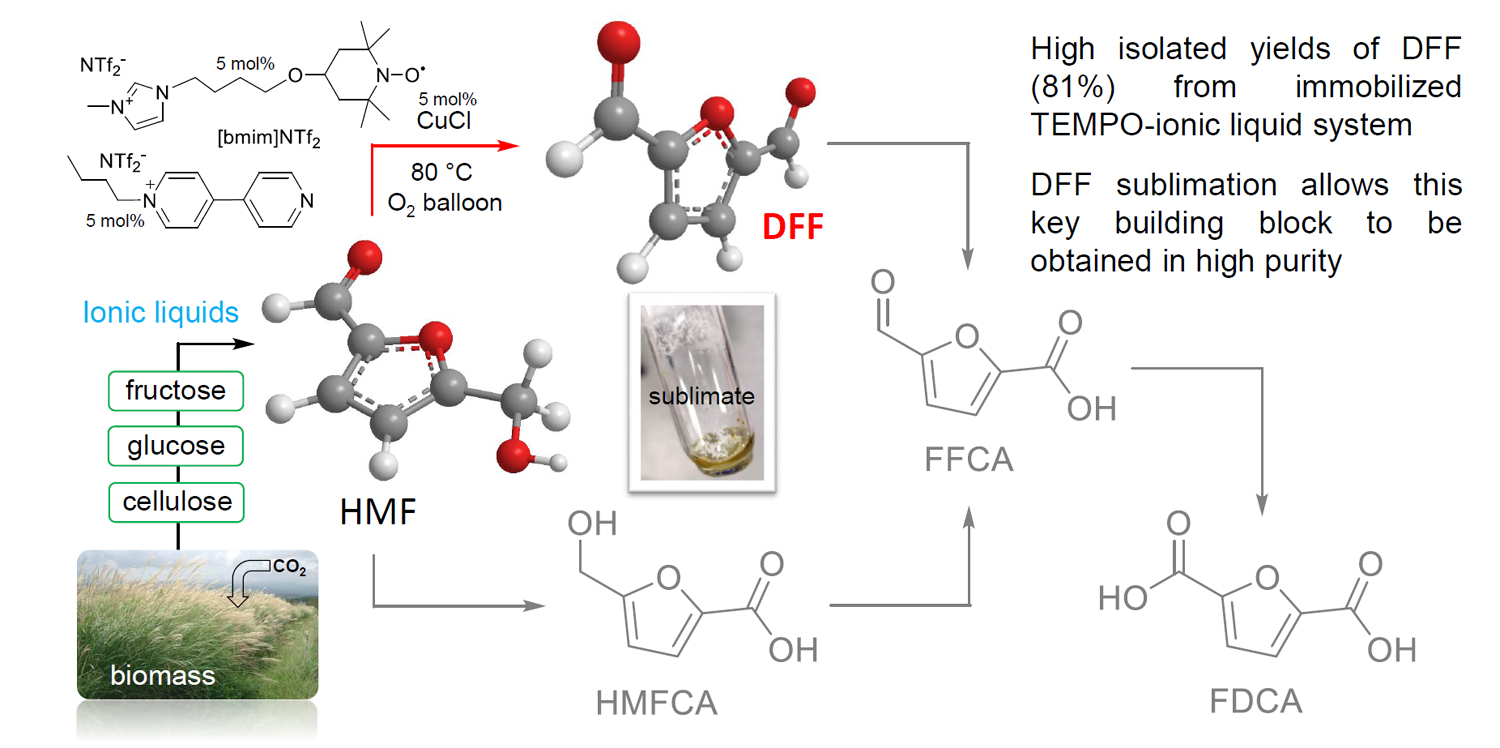
A. Al Ghatta, J. D. E. T. Wilton-Ely and J. P. Hallett, ACS Sustainable Chem. Eng. 2020, 8, 2462–2471 [DOI: 10.1021/acssuschemeng.9b06691]
For the biorefinery concept to be adopted, it is necessary to have an awareness of the variables that will underpin the generation of its products. One of the most attractive outputs from the transformation of biomass is 2,5-furandicarboxylic acid (FDCA), as shown at the top of the page. This can be used to make the polymer polyethylene furanoate (PEF), a superior alternative to the familiar PET plastic we use every day. Our recent study (Green Chemistry, 2021) assessed the variables involved in order to help direct research activities to address the weaknesses in the current pathways. A key aspect in the transformation of chemical reaction to large scale industrial processes is the separation of product mixtures, allowing recycling and valorisation of minor fractions (ACS Sustainable Chem. Eng., 2019).
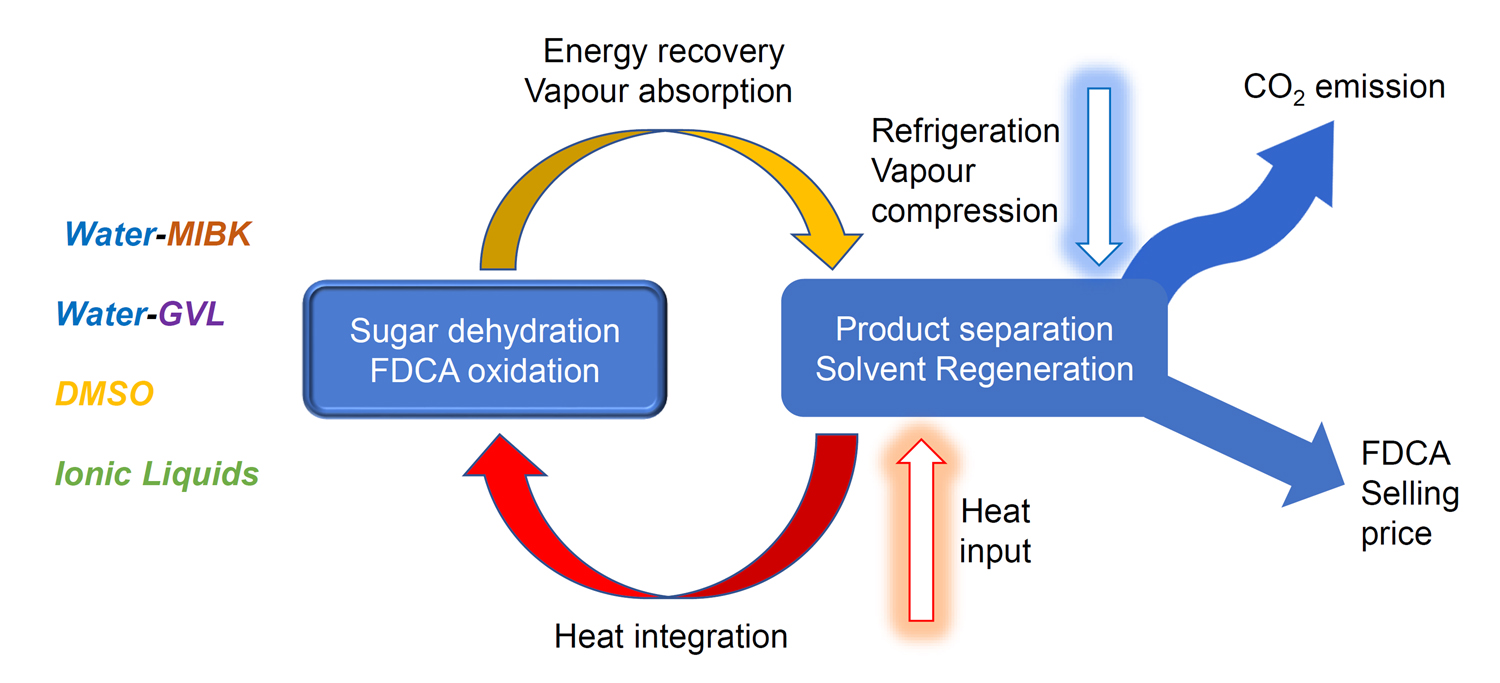
A. Al Ghatta, J. D. E. T. Wilton-Ely and J. P. Hallett, ACS Sustainable Chem. Eng. 2019, 7, 16483−16492 [DOI: 10.1021/acssuschemeng.9b03613]
A. Al Ghatta, J. D. E. T. Wilton-Ely, J. P. Hallett, Green Chem. 2021, 23, 1716-1733 [DOI: 10.1039/D0GC03991H]
The processing of biomass in ionic liquids has demonstrated many benefits compared to organic solvents. This includes the maximization of 5-hydroxymethylfurfural (HMF) yield from sugars through the suppression of byproducts, such as formic acid and levulinic acid. Inefficiencies still exist due to the low stability of HMF at high temperature, leading to side reactions which ultimately result in the undesirable formation of humins (polymeric carbonaceous compounds). Valorization of this side product is thus needed to improve the economics of the biorefinery and could lead to humins being viewed as valuable materials for various applications. The study summarised below provided insight into the control formation of these materials and demonstrated their potential use in the removal of antimony contamination from waste water.
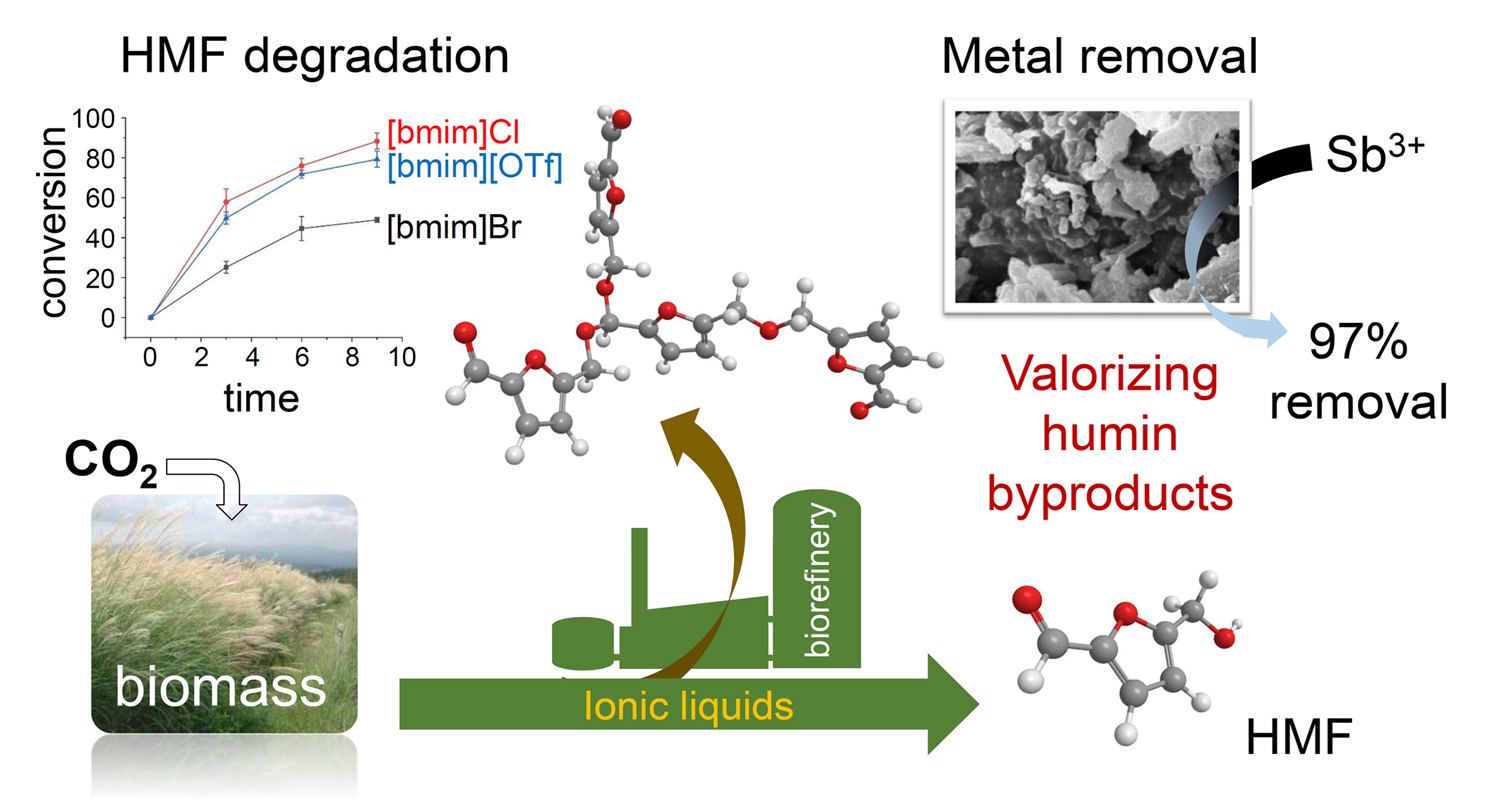
A. Al Ghatta, X. Zhou, G. Casarano, J. D. E. T. Wilton-Ely, J. P. Hallett, ACS Sustainable Chem. Eng. 2021, 9, 2212−2223 [DOI: 10.1021/acssuschemeng.0c07963]
 Research on gold nanoparticles has enjoyed rapid growth over the last two decades in the field of materials chemistry and, increasingly, in bioscience. We have a continuing interest in finding new ways of attaching metal complexes to the surface of gold nanoparticles for applications in medical imaging and therapy (see sections above). These materials can be treated in many ways as molecular compounds (solution NMR, IR, electrochemistry) yet offer a means to perform chemistry on a pre-organised surface of great surface area. Computational studies on these systems are being performed in collaboration with Prof. Fernando Bresme (Imperial Chemistry). We have also exploring magnetic nanoparticles with catalytic surface units and their application to 'click' chemistry in collaboration with Dr Silvia Diez-Gonzalez (Imperial Chemistry). This later work was featured on the Department's website.
Research on gold nanoparticles has enjoyed rapid growth over the last two decades in the field of materials chemistry and, increasingly, in bioscience. We have a continuing interest in finding new ways of attaching metal complexes to the surface of gold nanoparticles for applications in medical imaging and therapy (see sections above). These materials can be treated in many ways as molecular compounds (solution NMR, IR, electrochemistry) yet offer a means to perform chemistry on a pre-organised surface of great surface area. Computational studies on these systems are being performed in collaboration with Prof. Fernando Bresme (Imperial Chemistry). We have also exploring magnetic nanoparticles with catalytic surface units and their application to 'click' chemistry in collaboration with Dr Silvia Diez-Gonzalez (Imperial Chemistry). This later work was featured on the Department's website.
J. D. E. T. Wilton-Ely, Dalton Trans. 2008, 25; E. R. Knight, A. R. Cowley, G. Hogarth, J. D. E. T. Wilton-Ely, Dalton Trans. 2009, 607; E. R. Knight, N. H. Leung, A. L. Thompson, G. Hogarth, J. D. E. T. Wilton-Ely, Inorg. Chem. 2009, 48, 3866; E. R. Knight, N. H. Leung, Y. H. Lin, A. R. Cowley, D. J. Watkin, A. L. Thompson, G. Hogarth, J. D. E. T. Wilton-Ely, Dalton Trans. 2009, 3688; S. Naeem, A. Ribes, A. J. P. White, M. N. Haque, K. B. Holt, J. D. E. T. Wilton-Ely, Inorg. Chem. 2013, 52, 4700; J.-M. Collinson, J. D. E. T. Wilton-Ely, S. Diez-Gonzalez, Chem. Commun. 2013, 49, 11358; S. Naeem, S. Serapian, A. Toscani, A. J. P. White, G. Hogarth, J. D. E. T. Wilton-Ely, Inorg. Chem. 2014, 53, 2404; V. L. Hurtubise, J. McArdle, S. Naeem, A. Toscani, A. J. P. White, N. J. Long, J. D. E. T. Wilton-Ely, Inorg. Chem., 2014, 53, 11740; J.-M. Collinson, J. D. E. T. Wilton-Ely, S. Díez-González, Catal. Comm., 2016, 87, 78; J. A. Robson, F. Gonzalez de Rivera, K. A. Jantan, M. N. Wenzel, A. J. P. White, O. Rossell, J. D. E. T. Wilton-Ely, Inorg. Chem., 2016, 55, 12982; J.-M. Collinson, J. D. E. T. Wilton-Ely, S. Diez-Gonzalez, Catal. Commun. 2016, 87, 78.
Many years after the discovery that solutions of thiols organise themselves into Self-Assembled Monolayers (SAMs) on gold, these materials remain at the forefront of nanotechnology applications such as molecular electronics, (bio)sensors and catalytic supports. However, the quality of the monolayers formed can often be low with many defects and small domain sizes. We have made a contribution to this area through a collaboration with Prof. Manfred Buck at the University of St. Andrews and Dr Piotr Cyganik in Krakow. This focused on the preparation of new, functionalised thiols that form highly regular monolayers suitable for electronic applications.
P. Cyganik, M. Buck, J. D. E. T. Wilton-Ely, C. Woell, J. Phys. Chem. B 2005, 109, 10902; P . Cyganik, M. Buck, T . Strunskus, A. Shaporenko, J. D . E. T. Wilton-Ely, M. Z harnikov, C. Woell, J. Am. Chem. Soc. 2006, 128, 13868; C. Shen, M. Buck, J. D. E. T. Wilton-Ely, T. Weidner, M. Zharnikov, Langmuir 2008, 24, 6609.
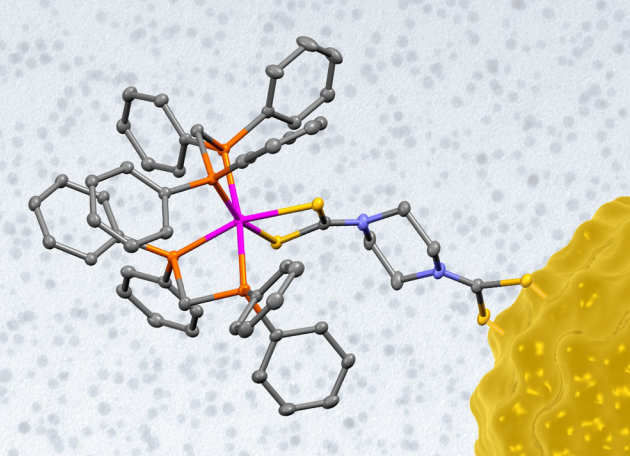
 We have a long-standing interest in the combination of interactions to form supramolecular assemblies. Hydrogen bonds are of similar energy (15 - 30 KJ/mol) to the gold-gold contacts observed in many solid state structures of monovalent gold complexes. In the past, we have been able to combine aurophilic and hydrogen bonding in the construction of supramolecular networks as shown in the tetramer on the right, held together by both types of interactions.
We have a long-standing interest in the combination of interactions to form supramolecular assemblies. Hydrogen bonds are of similar energy (15 - 30 KJ/mol) to the gold-gold contacts observed in many solid state structures of monovalent gold complexes. In the past, we have been able to combine aurophilic and hydrogen bonding in the construction of supramolecular networks as shown in the tetramer on the right, held together by both types of interactions.
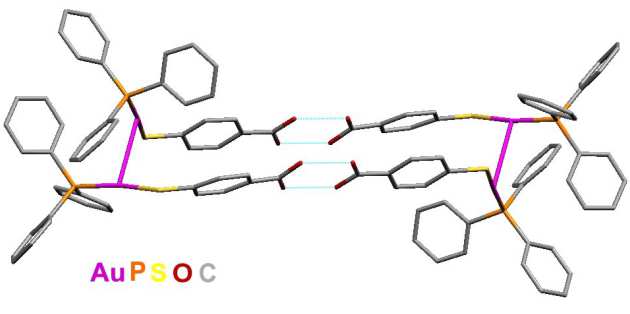
Dithiocarbamates form complexes with all the transition metals. We have developed methods of creating extended multimetallic arrays through bridging bis(dithiocarbamate) ligands in conjunction with Dr Graeme Hogarth at King's College London. These arrays have intersting properties both in terms of electrochemistry and as precursors for new materials through chemical vapour deposition. The example on the right shows an octahedral ruthenium centre attached to a square planar palladium unit.
 J. D. E. T. Wilton-Ely, A. Schier, N. W. Mitzel and H. Schmidbaur, J. Chem Soc., Dalton Trans., 2001, 1058; J. D. E. T. Wilton-Ely, D. Solanki, G. Hogarth, Eur. J. Inorg. Chem. 2005, 4027; J. D. E. T. Wilton-Ely, D. Solanki, E. R. Knight, K. B. Holt, A. L. Thompson, G. Hogarth, Inorg. Chem. 2008, 47, 9642; M. J. Macgregor, G. Hogarth, A. L. Thompson, J. D. E. T. Wilton-Ely, Organometallics 2009, 28, 197; E. R. Knight, N. H. Leung, A. L. Thompson, G. Hogarth, J. D. E. T. Wilton-Ely, Inorg. Chem. 2009, 48, 3866; E. R. Knight, N. H. Leung, Y. H. Lin, A. R. Cowley, D. J. Watkin, A. L. Thompson, G. Hogarth, J. D. E. T. Wilton-Ely, Dalton Trans. 2009, 3688; K. Oliver, A. J. P. White, G. Hogarth, J. D. E. T. Wilton-Ely, Dalton Trans., 2011, 40, 5852; S. Naeem, A. Ribes, A. J. P. White, M. N. Haque, K. B. Holt, J. D. E. T. Wilton-Ely, Inorg. Chem. 2013, 52, 4700; Y. H. Lin, L. Duclaux, F. Gonzàlez de Rivera, A. L. Thompson, J. D. E. T. Wilton-Ely, Eur. J. Inorg. Chem. 2014, 2065-2072; V. L. Hurtubise, J. M. McArdle, S. Naeem, A. Toscani, A. J. P. White, N. J. Long, J. D. E. T. Wilton-Ely, Inorg. Chem. 2014, 53, 11740-11748; A. Toscani, E. K. Heliovaara, J. B. Hena, A. J. P. White, J. D. E. T. Wilton-Ely, Organometallics 2015, 34, 494-505; R. Sherwood, F. Gonzàlez de Rivera, J. H. Wan, Q. Zhang, A. J. P. White, O. Rossell, G. Hogarth, J. D. E. T. Wilton-Ely, Inorg. Chem., 2015, 54, 4222; J. A. Robson, F. Gonzalez de Rivera, K. A. Jantan, M. N. Wenzel, A. J. P. White, O. Rossell, J. D. E. T. Wilton-Ely, Inorg. Chem., 2016, 55, 12982; A. Toscani, K. A. Jantan, J. B. Hena, J. A. Robson, E. J. Parmenter, V. Fiorini, A. J. P. White, S. Stagni, J. D. E. T. Wilton-Ely, Dalton Trans., 2017, 46, 5558; K. A. Jantan, J. M. McArdle, L. Mognon, V. Fiorini, L. A. Wilkinson, A. J. P. White, S. Stagni, N. J. Long, J. D. E. T. Wilton-Ely, New J. Chem., 2019, 43, 3199; L. Mognon, S. Richardson, G. Agonigi, T. Bond, F. Marchetti, J. D. E. T. Wilton-Ely, J. Organomet. Chem., 2019, 886, 9-12.
J. D. E. T. Wilton-Ely, A. Schier, N. W. Mitzel and H. Schmidbaur, J. Chem Soc., Dalton Trans., 2001, 1058; J. D. E. T. Wilton-Ely, D. Solanki, G. Hogarth, Eur. J. Inorg. Chem. 2005, 4027; J. D. E. T. Wilton-Ely, D. Solanki, E. R. Knight, K. B. Holt, A. L. Thompson, G. Hogarth, Inorg. Chem. 2008, 47, 9642; M. J. Macgregor, G. Hogarth, A. L. Thompson, J. D. E. T. Wilton-Ely, Organometallics 2009, 28, 197; E. R. Knight, N. H. Leung, A. L. Thompson, G. Hogarth, J. D. E. T. Wilton-Ely, Inorg. Chem. 2009, 48, 3866; E. R. Knight, N. H. Leung, Y. H. Lin, A. R. Cowley, D. J. Watkin, A. L. Thompson, G. Hogarth, J. D. E. T. Wilton-Ely, Dalton Trans. 2009, 3688; K. Oliver, A. J. P. White, G. Hogarth, J. D. E. T. Wilton-Ely, Dalton Trans., 2011, 40, 5852; S. Naeem, A. Ribes, A. J. P. White, M. N. Haque, K. B. Holt, J. D. E. T. Wilton-Ely, Inorg. Chem. 2013, 52, 4700; Y. H. Lin, L. Duclaux, F. Gonzàlez de Rivera, A. L. Thompson, J. D. E. T. Wilton-Ely, Eur. J. Inorg. Chem. 2014, 2065-2072; V. L. Hurtubise, J. M. McArdle, S. Naeem, A. Toscani, A. J. P. White, N. J. Long, J. D. E. T. Wilton-Ely, Inorg. Chem. 2014, 53, 11740-11748; A. Toscani, E. K. Heliovaara, J. B. Hena, A. J. P. White, J. D. E. T. Wilton-Ely, Organometallics 2015, 34, 494-505; R. Sherwood, F. Gonzàlez de Rivera, J. H. Wan, Q. Zhang, A. J. P. White, O. Rossell, G. Hogarth, J. D. E. T. Wilton-Ely, Inorg. Chem., 2015, 54, 4222; J. A. Robson, F. Gonzalez de Rivera, K. A. Jantan, M. N. Wenzel, A. J. P. White, O. Rossell, J. D. E. T. Wilton-Ely, Inorg. Chem., 2016, 55, 12982; A. Toscani, K. A. Jantan, J. B. Hena, J. A. Robson, E. J. Parmenter, V. Fiorini, A. J. P. White, S. Stagni, J. D. E. T. Wilton-Ely, Dalton Trans., 2017, 46, 5558; K. A. Jantan, J. M. McArdle, L. Mognon, V. Fiorini, L. A. Wilkinson, A. J. P. White, S. Stagni, N. J. Long, J. D. E. T. Wilton-Ely, New J. Chem., 2019, 43, 3199; L. Mognon, S. Richardson, G. Agonigi, T. Bond, F. Marchetti, J. D. E. T. Wilton-Ely, J. Organomet. Chem., 2019, 886, 9-12.
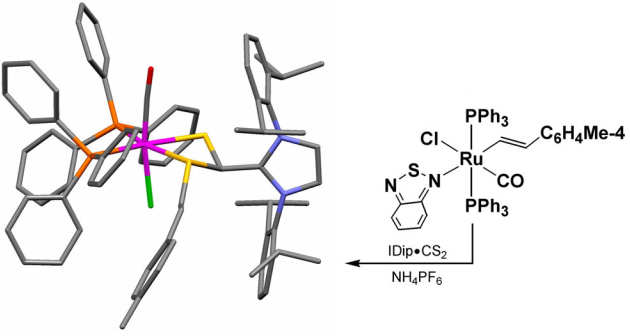 Sulfur ligands underpin many of the activities in the group and part of the origins of this interest come from a collaboration with Prof. Lionel Delaude, University of Liege, Belgium. N-heterocylic carbenes have become powerful ligands in their own right but they can also be used to form zwitterionic ligand systems of the form NHC.C(A)S where A = O, C, NR. We have been exploring the reactivity of these ligands with ruthenium, palladium and gold and are now optimising their use as ligands to support catalytic oxidative C-H functionalisation.
Sulfur ligands underpin many of the activities in the group and part of the origins of this interest come from a collaboration with Prof. Lionel Delaude, University of Liege, Belgium. N-heterocylic carbenes have become powerful ligands in their own right but they can also be used to form zwitterionic ligand systems of the form NHC.C(A)S where A = O, C, NR. We have been exploring the reactivity of these ligands with ruthenium, palladium and gold and are now optimising their use as ligands to support catalytic oxidative C-H functionalisation.
S. Naeem, A. L. Thompson, L. Delaude, J. D. E. T. Wilton-Ely, Chem. Eur. J. 2010, 16, 10971; S. Naeem, L. Delaude, A. J. P. White, J. D. E. T. Wilton-Ely Inorg. Chem. 2010, 49, 1784; S. Naeem, A. L. Thompson, A. J. P. White, L. Delaude, J. D. E. T. Wilton-Ely, Dalton Trans. 2011, 40, 3737; E. Y. Chia, S. Naeem, A. J. P. White, L. Delaude, J. D. E. T. Wilton-Ely, Dalton Trans. 2011, 40, 6645; M. J. D. Champion, R. Solanki, L. Delaude, A. J. P. White, J. D. E. T. Wilton-Ely, Dalton Trans. 2012, 41, 12386