Alternative metal-ion batteries, such as Sodium, Potassium and Aluminium, have shown potential as cost-effective and sustainable successors to lithium-ion batteries. In addition to the metals’ high abundance in the Earth’s crust, there are many advantages to exploring these battery designs. Sodium-ion batteries offer reduced cost due to cheaper equivalent sodium electrolytes and economical aluminium current collectors, instead of copper as in lithium-ion batteries. Lithium- and sodium-ion battery processing systems are also identical, making NIB production a ‘drop-in’ technology. Potassium-ion batteries are promising for high power density due to the high ionic conductivity and small stokes radius of potassium ions. Potassium-ion battery electrolytes are also cheaper than lithium-based electrolyte materials. In rechargeable aluminium batteries, the trivalent aluminium ion, promises high volumetric energy density. The stability of aluminium metal and the low flammability of ionic liquid electrolytes, typically employed in aluminium dual-ion batteries, offer improved operational safety.
As part of the ESE group, the Titirici group at the Department of Chemical Engineering explores a range of the challenges facing alternative metal-ion batteries. While graphite, the standard LIB anode material, shows little capacity for sodium-ion storage, the group studies the mechanism of Na-ion storage in other carbonaceous materials, by tuning their structure (e.g. defects, porosity, graphitisation), and relates this information to the electrochemical performance. In potassium-ion batteries, ion intercalation into graphite causes significant volume expansion, such that research into different anode materials, such as soft carbons and hard carbons, is valuable to improve KIB cyclability. Regarding aluminium batteries, the group is interested in developing highly graphitic cathode materials to improve intercalation and increase the achievable energy density, while investigating cheaper and less corrosive electrolytes. More information on alternative metal-ion and metal dual-ion batteries can be found on their website. Click to go to the Titirici group
Recent publications 2020 - to date
Xu Z, Guo Z, Madhu R, Xie F, Chen R, Wang J, Tebyetekerwa M, Hu Y, Titirici M, 2021, Homogenous metallic deposition regulated by defect-rich skeletons for sodium metal batteries, Energy & Environmental Science
Pan W, Zhao Y, Mao J, Wang Y, Zhao X, Leong KW, Luo S, Liu X, Wang H, Xuan J, Yang S, Chen Y, Leung DYC, 2021, High-Energy SWCNT Cathode for Aqueous Al-Ion Battery Boosted by Multi-Ion Intercalation Chemistry Adv. Energy Mater., Pages: 2101514.
Pan W, Wang Y, Zhao X, Zhao Y, Liu X, Xuan J, Wang H, Leung DYC, 2021, High-Performance Aqueous Na–Zn Hybrid Ion Battery Boosted by “Water-In-Gel” Electrolyte, Adv. Funct. Mater., Vol: 31, Pages:2008783.
Guo Z, Xu Z, Xie F, Feng J, Titirici M, 2021, Strategies for High Energy Density Dual-Ion Batteries Using Carbon-Based Cathodes, Adv. Energy Sustainability Res. 2100074.
Pan W, Wang Y, Zhao X, Zhao Y, Liu Y, Xuan J, Wang H, Leung DYC, 2021, High-Performance Aqueous Na–Zn Hybrid Ion Battery Boosted by “Water-In-Gel” Electrolyte, Advanced Functional Material, pages: 2008783
Titirici MM, Alptekin H, Au H, Jensen ACS, Olsson E, Goktas M, Headen TF, Adelhelm P, Cai Q, Drew AJ, 2020, Sodium storage mechanism investigations through structural changes in hard carbons. ACS Applied Energy Materials, Vol: 3 (10), Pages: 9918–9927.
Zhang S, Teck AA, Guo Z, Xu Z, Titirici M, 2021, Carbon Composite Anodes with Tunable Microstructures for Potassium‐Ion Batteries. Batteries & Supercaps, batt.202000306.
Au H, Alptekin H, Jensen A, Olsson E, A O’Keefe C, Smith T, Crespo-Ribadeneyra M, Headen T, Grey C, Cai Q, Drewb A, Titirici M, 2020, A revised mechanistic model for sodium insertion in hard carbons, Energy & Environmental Science.
Alptekin H, Au H, Jensen A, Olsson E, GOKTAS M, Headen TF, Adelhelm P, Cai Q, Drew AJ, Titirici MM, 2020, Sodium Storage Mechanism Investigations Through Structural Changes in Hard Carbons, ACS Applied Energy Materials.
Jensen A, Au H, Gärtner S, Titirici M, Drew A, 2020, Solvation of NaPF6 in diglyme solution for battery electrolytes, Batteries & Supercaps.
Our new paper on a revised mechanistic model for sodium insertion in hard carbons
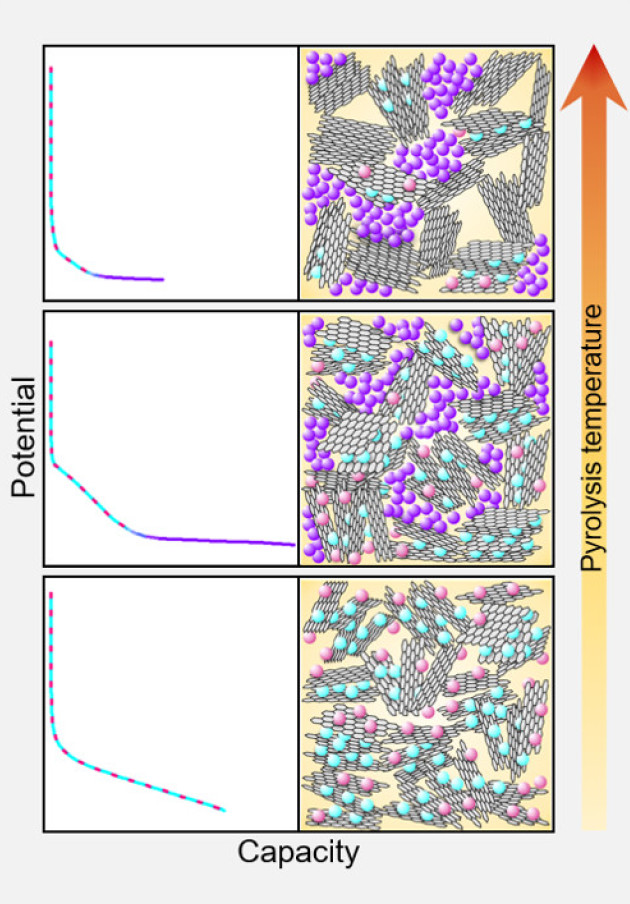 Au H, Alptekin H, Jensen A, Olsson E, A O’Keefe C, Smith T, Crespo-Ribadeneyra M, Headen T, Grey C, Cai Q, Drewb A, Titirici M, 2020, A revised mechanistic model for sodium insertion in hard carbons, Energy & Environmental Science.
Au H, Alptekin H, Jensen A, Olsson E, A O’Keefe C, Smith T, Crespo-Ribadeneyra M, Headen T, Grey C, Cai Q, Drewb A, Titirici M, 2020, A revised mechanistic model for sodium insertion in hard carbons, Energy & Environmental Science.
Hard carbons have shown considerable promise as anodes for emerging sodium-ion battery technologies. Current understanding of sodium-storage behaviour in hard carbons attributes capacity to filling of graphitic interlayers and pores, and adsorption at defects, although there is still considerable debate regarding the voltages at which these mechanisms occur. Here, ex-situ 23Na solid-state NMR and total scattering studies on a systematically tuned series of hard carbons revealed the formation of increasingly metallic sodium clusters in direct correlation to the growing pore size, occurring only in samples which exhibited a low voltage plateau. Combining experimental results with DFT calculations, we propose a revised mechanistic model in which sodium ions store first simultaneously and continuously at defects, within interlayers and on pore surfaces. Once these higher energy binding sites are filled, pore filling occurs during the plateau region, where the densely confined sodium takes on a greater degree of metallicity.