Solid oxide fuel cells are a type of fuel cell which have exceptional efficiency and the ability to run on fuels other than hydrogen. Work in the ESE group looks at improving the electrodes of these fuel cells by studying and improving the materials used in them and their structures. This includes developing new materials, studying the chemical and electrochemical characteristics of new and existing materials, developing new fabrication techniques to create novel microstructures and multiscale modelling to provide guidance on desired structures and materials properties and the impact of new materials and structures at the stack level.
Current projects
- Characterisation of gas-solid interactions in fuel cell electrode materials
- Investigating the properties and degradation of solid-solid interfaces in solid oxide fuel cells
- Design of an electrospun fibre-based structure for Ni/CGO SOFCs anode
Lead researcher: Dr Paul Boldrin
PI: Prof Nigel Brandon
Funding:
- EPSRC UK grant, Electrodes by Design - Microstructural Engineering of High Performance Electrodes for Solid Oxide Fuel Cells (EP/M014045/1 )
- EPSRC UK grant, Energy storage for low carbon grids (EP/J021199/1)
At the heart of processes occurring in fuel cells of all types is the interactions between the fuel and oxidant gases and the electrode materials. While electrode performance can be measured using techniques such as electrochemical impedance spectroscopy (EIS), in these many factors such as conductivity and microstructure are bundled in together with reaction rates meaning that separate contributions to performance are not able to be assessed and possible routes for improvement are obscured. Ex situ techniques looking directly at the gas-solid interactions in fuel cells are thus of great benefit in helping to disentangle the different contributions to performance and identify potential opportunities.
The work in our group encompasses a range of methods involving passing gases over solids and broadly fit into two groups – pulse techniques, where pulses of a gas are passed over a solid and temperature-programmed techniques, where the changes in the interactions between gas and solid are measured as a function of temperature. The former, depending on how it is used, can give precise quantification of surface areas or information about reaction mechanisms, while the latter can give quantifications of kinetic parameters such as reaction orders and energies of adsorption, as well as information on reaction mechanisms. These results can then be used with other ex-situ techniques such as XPS and Raman to obtain a more complete picture of gas-solid interactions.
We have used these techniques to study materials used in solid oxide fuel cells, polymer electrolyte fuel cells and redox flow batteries, as well as materials used in related non-electrochemical systems such as membrane reactors, chemical looping combustion and thermochemical water splitting. Below is an example relevant to solid oxide fuel cells and chemical looping combustion
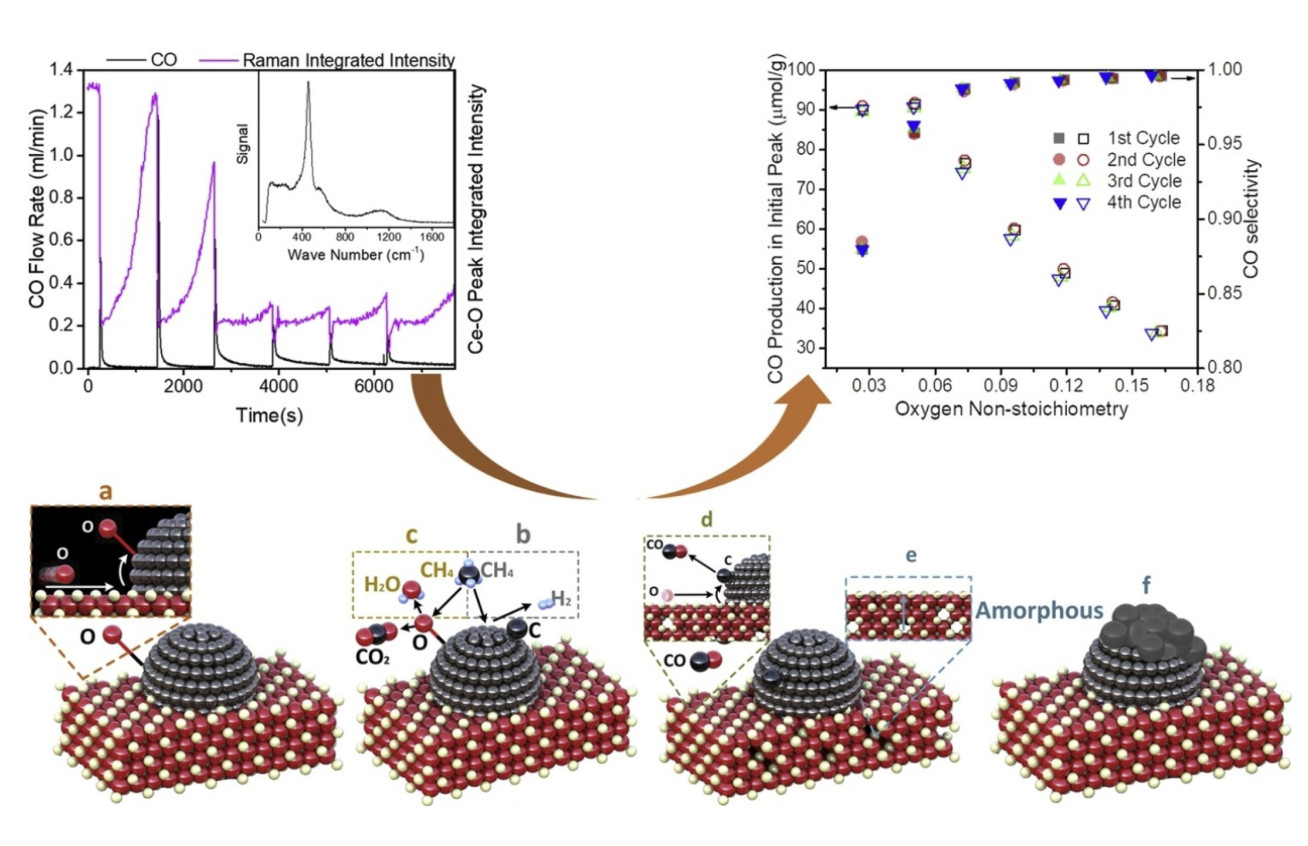
The mechanism of methane oxidation on Ni/CGO SOFC anodes: An example of a pulsed technique was used to give insights into the mechanism of methane oxidation on Ni/CGO (Figure 1), a common electrode material in solid oxide fuel cells (SOFCs). SOFCs possess an advantage compared to lower temperature fuel cells such as PEFCs in that they can use methane as a fuel directly. However, the introduction of methane causes problems such as carbon deposition which can lead to electrode failure, as well as complicating the reaction mechanisms meaning it can be difficult to get useful insights from EIS. We performed a series of experiments pulsing methane over Ni/CGO at 600 °C, looking at the gases produced. Because pulse techniques allow precise quantification of the volumes of gases emitted, we were able to deduce that the reaction mechanism is extremely sensitive to the stoichiometry of the CGO, with total oxidation dominating at high oxygen stoichiometries and partial oxidation dominating at lower stoichiometries. In addition, we were able to observe changes in the gases produced with ageing, which we are currently modelling in order to obtain microstructural information.
Collaborators:
- Department of Physics: Lesley Cohen
- University of Twente: Dr Aayan Banerjee
- Boeing
- Ceres Power
Publications:
-
Ouyang M, Boldrin P, Maher RC, Chen X, Liu X, Cohen LF, Brandon NP, 2019, A mechanistic study of the interactions between methane and nickel supported on doped ceria, Applied Catalysis B: Environmental, Volume 248, Pages 332-340
Lead researchers: Mr Liam Yasin
PI: Dr Sam Cooper
Funding:
- Science and Solutions for a Changing Planet Doctoral Training Programme (SSCP DTP)
Our project aims to elucidate the behaviour of interfaces in Solid Oxide Fuel Cells (SOFC) and other electrochemical devices. By using Isotope Exchange Depth Profiling (IEDP) with Secondary Ion Mass Spectrometry (SIMS), the diffusion behaviour of oxygen ions in a SOFC and its interfaces can be directly probed. This allows for the extraction of materials properties in the cell and how these change after being exposed to various degradation conditions. In this way, the influence of interfaces on the overall cell performance and degradation can be determined, which might help improve the lifetime of these fuel cell systems.
In order to accurately extract information about the properties of the material from the experimental data, a finite-difference diffusion model, which takes into account the presence of interfaces in the cell, is employed. This allows for the quantification of potential interfacial resistance effects. This project is partially undertaken in collaboration with Ceres Power, a UK based fuel cell manufacturer.
Collaborators:
- Department of Materials: Dr Ainara Aguadero (co-supervisor)
- Ceres Power
Publications:
-
To be updated.

Lead researchers: Dr Mengzheng Ouyang
PI’s: Prof Nigel Brandon
Funding:
- EPSRC UK grant, Hydrogen and Fuel Cells Hub Extension (H2FC SUPERGEN, EP/P024807/1)
- EPSRC UK grant, Electrodes by Design - Microstructural Engineering of High Performance Electrodes for Solid Oxide Fuel Cells (EP/M014045/1)
In conventional SOFCs Ni/yttrium stabilised zirconia (YSZ) anode, electrochemical oxidation of hydrogen is generally regarded to happen on the triple-phase boundary (TPB) of electron conducting phase (Ni), ion conducting phase (YSZ) and pores. The replacement off YSZ by doped ceria has been a trend in the recent years. Because doped ceria is an exceptional electrochemical catalyst itself, in Ni/gadolinium doped ceria (CGO) SOFCs anodes, the active sites extends from TPB to dual-phase boundary of CGO. However, the microstructure designs of the anode are still towards maximizing the TPB, which has not been adapted to the change of materials property.
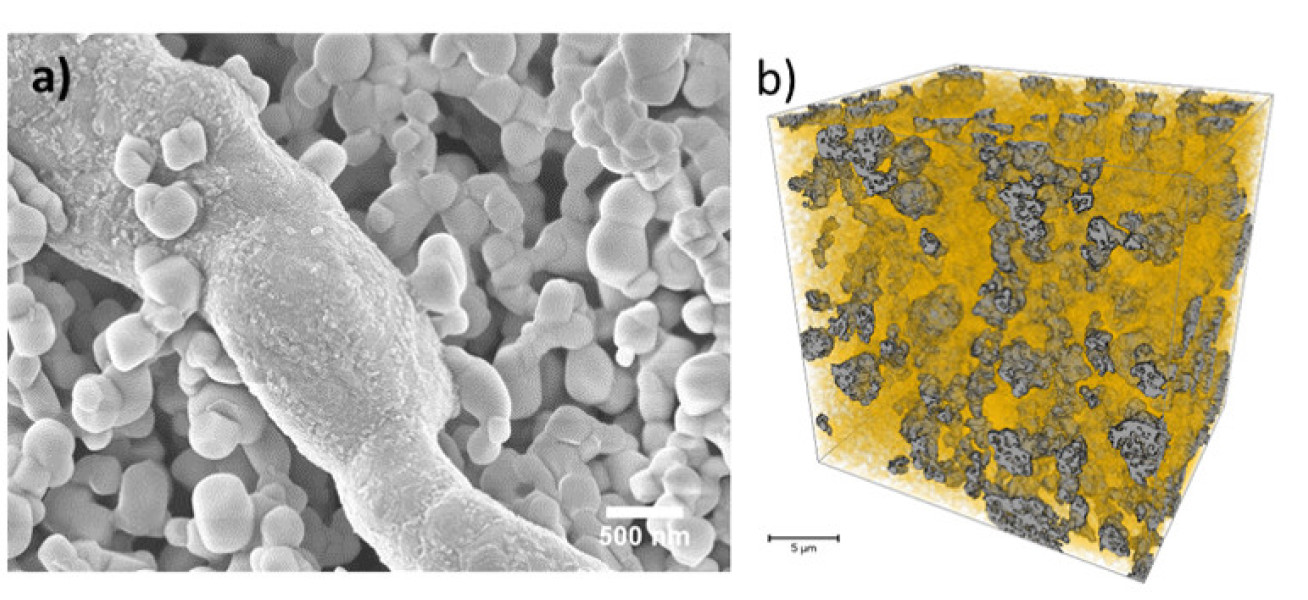
In this project, we designed a novel Ni/CGO electrode structure with maximized CGO DPB, consisting of continuous Ni fibres made by electrospinning and CGO particles introduced by facile tape-casting. As shown in Figure 1a, in the middle is the Ni fibre with diameter of 800 μm. The smaller particles (~200 nm) surrounding it is CGO. In Figure 1b shows that CGO (orange phase) filled all the pores of Ni fibre network (grey phase).
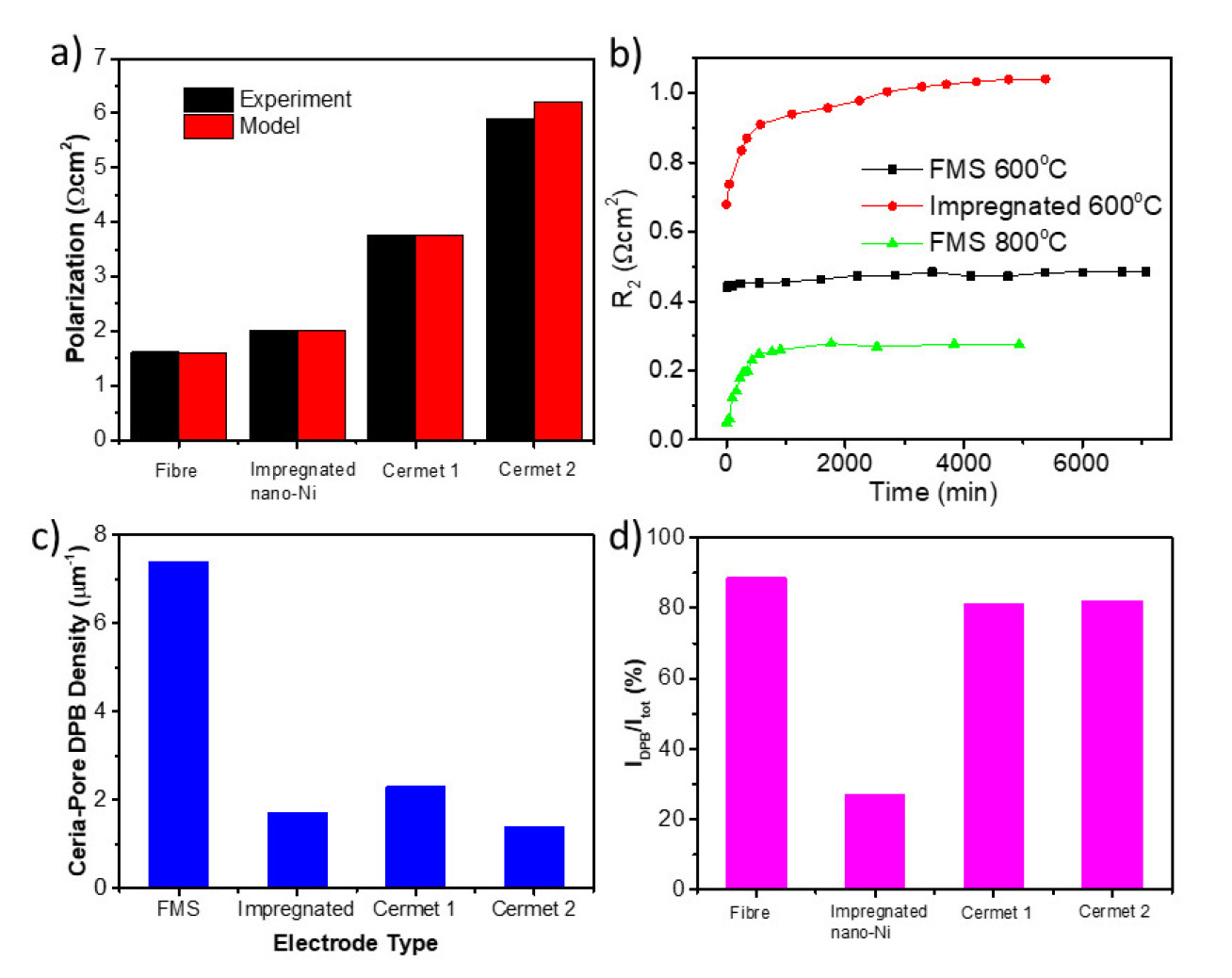
The as-made fibre-matrix structure (FMS) shows lower polarisation resistance (Figure 2a). and better thermal stability (Figure 2b). compared with conventional anode structures.
We also analysed four Ni/CGO anodes with different structures using a physical model which enables us to quantitatively correlate the microstructure parameters with electrochemical performance. The DPB of FMS is 3 times of conventional structures (Figure 2c). In FMS electrodes, 90% of electrochemical activity comes from DPB (Figure 2d), while in impregnated Ni/CGO, only 27% activity is from DPB. Also, the rate-limiting step of the anode reaction is determined to be the electrochemical oxidation of hydrogen.
Because the FMS structure is made of commercial materials, there is still large rooms for performance improvement. In the current step, we are trying to increase the CGO’s surface area by using impregnated CGO rather than tape-casted CGO.
Collaborators:
- ESE group - Dyson School of Engineering: Dr Samuel Cooper
- Department of Computing: Dr Yufei Wu
- Beihang University: Dr Xinhua Liu
- University of Pisa: Dr Antonio Bertei
- Kyoto University: Dr Masashi Kishimoto
Publications:
- Ouyang M , Bertei A, Cooper SJ, Wu Y, Boldrin P, Liu X, Kishimoto M, Wang H, Marlow MN, Chen J, Chen X, Xia Y, Wu B, Brandon NP, 2020, Model-guided Design of a High Performance and Durability Ni Nanofiber/ceria Matrix Solid Oxide Fuel Cell Electrode, Journal of Energy Chemistry, Vol: 56, Pages: 98-112.
- Ouyang M, Bertei A, Cooper SJ, Wu Y, Liu X, Boldrin P, Kishimoto M, Wu B, Brandon NP, 2019, Design of Fibre Ni/CGO Anode and Model Interpretation, ECS Transactions, Vol: 91, Pages: 1721-1739.
Recent publications 2020 - to date
Cavallaro A, Wilson G, Kerherve G, Cali E, van den Bosh C, Boldrin P, Payne D, Skinner SJ, Aguadero A, 2021, Analysis of H2O-induced surface degradation in SrCoO3-derivatives and its impact on the redox kinetics, J. Mater. Chem. A.
Wehrle L, Wang Y, Boldrin P, Brandon NP, Deutschmann O, Banerjee A, 2021, Optimizing Solid Oxide Fuel Cell Performance to Re-evaluate Its Role in the Mobility Sector, ACS Environ. Au.
Chen J, Ouyang M, Boldrin P, Atkinson A, Brandon NP, 2020, Understanding the coarsening and degradation in a nanoscale nickel gadolinia-doped-ceria electrode for high-temperature applications, ACS Applied Materials & Interfaces, Vol:12, ISSN:1944-8244, Pages:47564-47573
Ouyang M, Bertei A, Cooper SJ, Wu Y, Boldrin P, Liu X, Kishimoto M, Wang H, Naylor Marlow M, Chen J, Chen X, Xia Y, Wu B, Brandon NP, 2020, Model-guided design of a high performance and durability Ni nanofiber/ceria matrix solid oxide fuel cell electrode, Journal of Energy Chemistry, In Press.
Li T, Lu X, Rabuni MF, Wang B, Farandos NM, Kelsall GH, Brett DJL, Shearing PR, Ouyang M, Brandon NP, Li K, 2020, High-performance fuel cell designed for coking-resistance and efficient conversion of waste methane to electrical energy, Energy & Environmental Science, Vol: 13, Pages: 1879-1887
We wrote the book!
 Solid Oxide Fuel Cell Lifetime and Reliability: Critical Challenges in Fuel Cells, 2017, 1st edn., Edited by N. P. Brandon, E. Ruiz-Trejo, and P. Boldrin. London.
Solid Oxide Fuel Cell Lifetime and Reliability: Critical Challenges in Fuel Cells, 2017, 1st edn., Edited by N. P. Brandon, E. Ruiz-Trejo, and P. Boldrin. London.
This book presents in one volume the most recent research that aims at solving key issues for the deployment of SOFC at a commercial scale and for a wider range of applications. To achieve that, authors from different regions and backgrounds address topics such as electrolytes, contaminants, redox cycling, gas-tight seals, and electrode microstructure. Lifetime issues for particular elements of the fuel cells, like cathodes, interconnects, and fuel processors, are covered as well as new materials. They also examine the balance of SOFC plants, correlations between structure and electrochemical performance, methods for analysis of performance and degradation assessment, and computational and statistical approaches to quantify degradation.
