ElectroCardioMaths Programme
RIPPLE MAPPING – invented, clinically-developed and multicentre trial led by Imperial ECM collaboration (Linton/Kanagaratnam)
The ElectroCardioMaths programme, the centrepiece of the recently designated Imperial Centre for Cardiac Engineering, is a multidisciplinary initiative bringing together research leaders from clinical electrophysiology, biological sciences and physical sciences to address key challenges in the diagnosis and treatment of complex cardiac arrhythmias. Complex arrhythmias include both the most common heart rhythm disturbance, atrial fibrillation (AF), which is increasingly prevalent with age – affecting 10% of the over sixties and the most important cause of stroke worldwide – and the heart rhythm disturbance responsible for half of all cardiac deaths.
The diverse nature of the group, which has received funding from EPSRC, MRC, BHF and Wellcome Trust, enables a distinctive multi-faceted approach to addressing these problems, providing unique insight into the initiation and perpetuation of arrhythmias and subsequently the development of novel therapeutic solutions. The programme spans an integrated spectrum of research, translating novel mathematical, engineering and biological techniques into improvements in clinical practice and patient care. With extensive combined expertise in machine learning, medical imaging, computational modelling, signal analysis and a range of cell-scale and tissue-scale laboratory techniques, the ElectroCardioMaths programme is able to deliver a true “chip to cell to bedside” approach. Since its inception in 2010, the group has published over 40 papers and has generated several patent applications and technologies currently being tested or incorporated into systems in clinical use.
Solving Complex Arrhythmogenesis with Machine Learning
In 2017 the ElectroCardioMaths programme expanded substantially into using modern machine learning techniques. Our machine learning team (comprising 3 full-time post-docs and 2 PhD students) focus specifically on leveraging the rich and complex architectural and electrophysiological myocardial mapping data to identify electro-architectural signatures in complex electrograms, solve the relationship between myocardial architecture and electrophysiology, and hence develop non-invasive MRI-based electrophysiological diagnosis, risk stratification and targeting.
Non-invasive ElectroMechanical Mapping, targeting and delivery of ablation totally non-invasively.
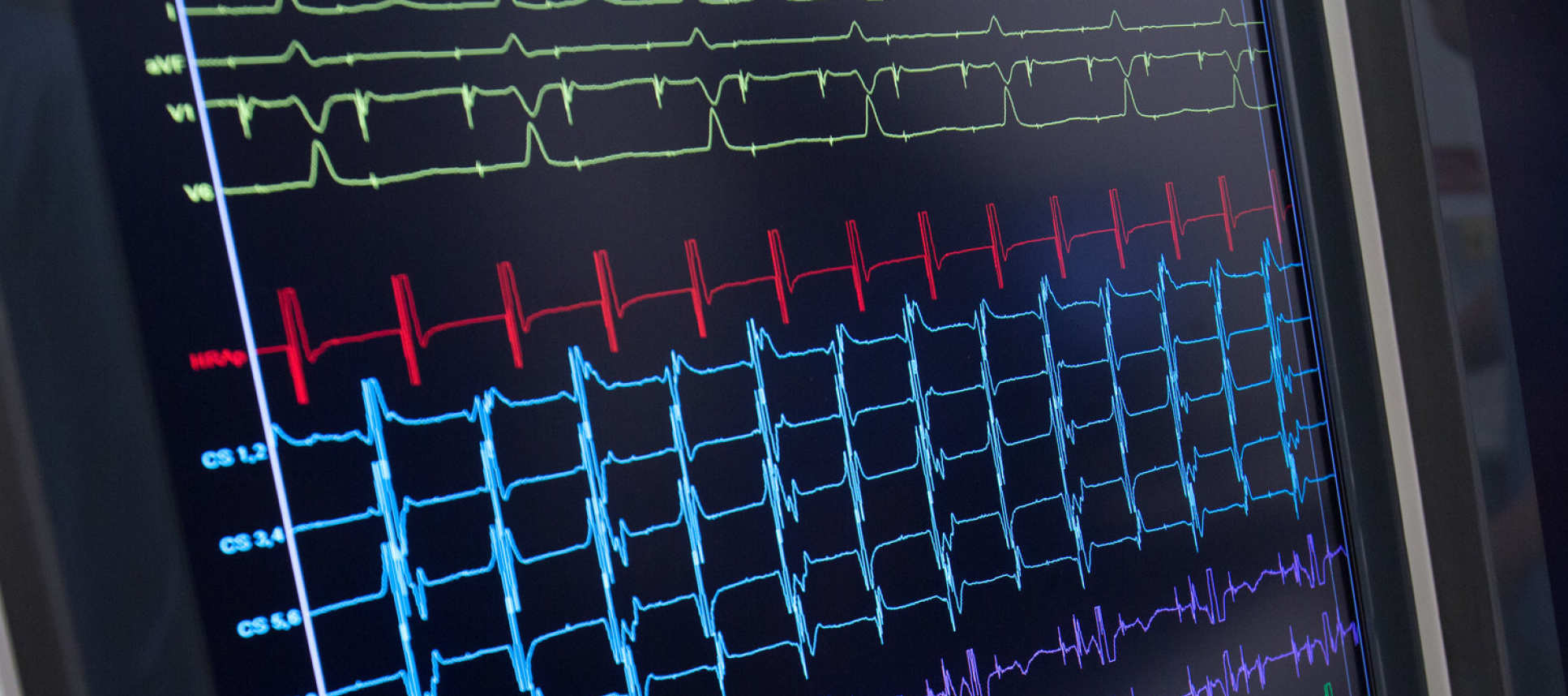
We are drawing on our unique combination of expertise in both non-invasive MRI-based mapping of myocardial architecture and wearable body-surface ECG-imaging based electrophysiological mapping individually to develop the science of targeting and applying external-beam radiotherapy for ablation of arrhythmic myocardial targets. Proof of concept has recently been described by others, but the unique combination of true multidisciplinary understanding of the subject combining expertise in modelling, machine learning, multiscale invasive & non-invasive electro-architectural mapping makes ElectroCardioMaths ideally placed to do the iterative translational and reverse-translational work to refine the science evolve for systematic development and scaling this clinical approach and the underlying technology.
A truly integrative approach
Our mission centres on combining and leveraging all aspects of group, for example:
- understanding the structural and functional relationships in myocardium, by integrating in-vivo, ex-vivo, in-vitro and in-silico approaches;
- using cutting-edge numerics to develop high-performance computer models of the heart, personalised through the integration of electroanatomic and imaging data, to aid diagnosis and improve the accuracy of intervention;
- developing new approaches for predictive modelling of atrial fibrillation using deep learning;
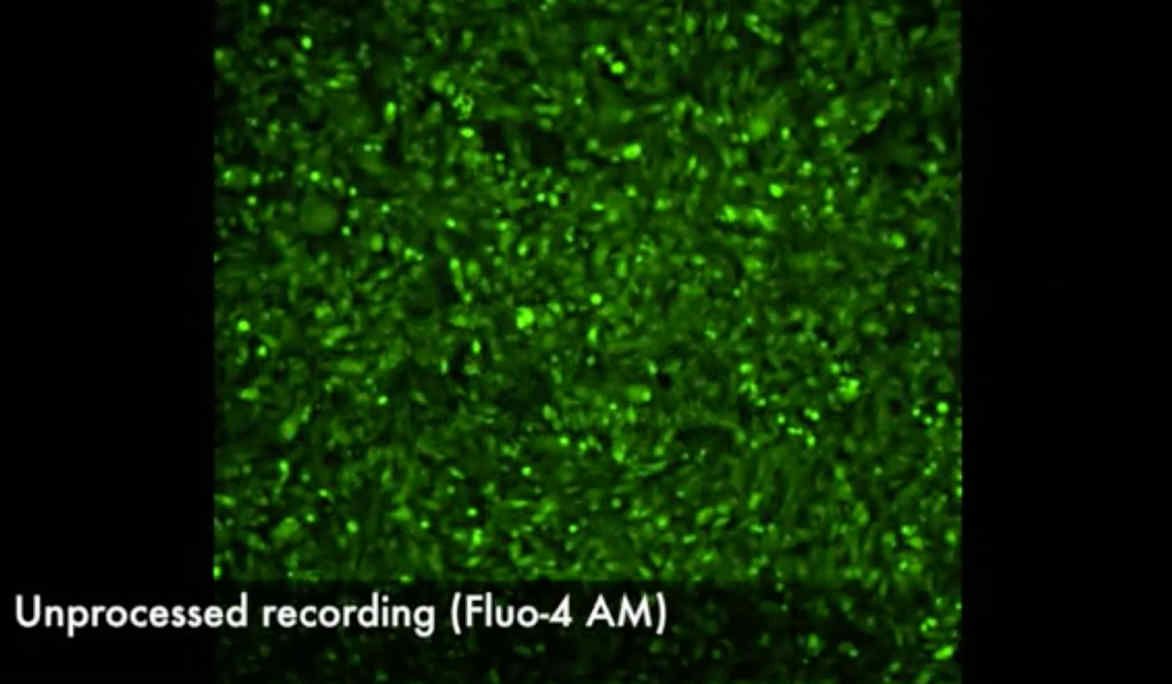
- designing novel algorithms for analysing the spatiotemporal dynamics of action potential propagation using optical mapping and electro-anatomic data modalities;
- creating novel approaches for analysing electro-anatomic and imaging data to support interpretation and clinical diagnosis.
A cross-faculty research group
The group includes academics from the NHLI, Department of Bioengineering, Department of Aeronautics, Department of Computing, Department of Physics and the Data Science Institute. Read more about them and their individual work below:
Professor Anil A Bharath
Professor Anil A Bharath
Academic Director (Singapore)
Dr Chris Cantwell
Dr Chris Cantwell
Senior Lecturer in Aeronautics
Dr Rasheda A Chowdhury

Dr Rasheda A Chowdhury
Advanced Research Fellow
Professor Kim Christensen

Professor Kim Christensen
Professor of Theoretical Physics
Professor Prapa Kanagaratnam
Professor Prapa Kanagaratnam
Professor of Cardiology
Dr Nick Linton

Dr Nick Linton
Clinical Senior Lecturer
Dr Fu Siong Ng

Dr Fu Siong Ng
Reader in Cardiac Electrophysiology
Professor Declan P O'Regan

Professor Declan P O'Regan
Professor of Imaging Sciences
Professor Nicholas S Peters

Professor Nicholas S Peters
Professor of Cardiac Electrophysiology
Professor Daniel Rueckert
Professor Daniel Rueckert
Professor of Visual Information Processing
Professor Spencer J Sherwin FREng

Professor Spencer J Sherwin FREng
Head of the Department of Aeronautics