Community Jameel fund drives high-impact COVID-19 projects

A new fund supported by Community Jameel is accelerating projects aimed at protecting the world from COVID-19 and future infectious disease threats.
Imperial College London and Community Jameel have announced the first projects to receive funding from The Jameel Fund for Infectious Disease Research and Innovation (“The Jameel Fund”) - which provides grants of up to $65,000 for short-term, high-impact projects that advance our ability to understand, prevent, diagnose, and treat infectious diseases.
This first round of funding is awarded to projects focused on the transmission of coronaviruses – including COVID-19, SARS and MERS - and the pathogenesis of these diseases, or how they develop.
The first projects to receive funding from the Jameel Fund include:
- Creating a 3D facial scanning mobile app for mass customisation of respiratory protective equipment (RPE) (e.g. masks and face shields)
- Developing rapid diagnostic screening
- Understanding severe illness in children linked to COVID-19
- Exploring if DNA sequencing technology can be engineered to detect new variants
The fund is supported by a grant from Community Jameel, the international organisation supporting science, evidence, data and technology.
Customisable masks
Dr Connor Myant, from the Dyson School of Design Engineering, and in partnership with PolyMetrix, is developing a 3D facial scanning mobile phone app to enable the mass customisation of respiratory protective equipment (RPE) like masks and face shields.
RPE can slow the transmission of coronaviruses like COVID-19, but this relies on the equipment fitting the wearer properly. Prolonged use of incorrectly fitting RPE can also cause discomfort and lead to rashes and blistering.
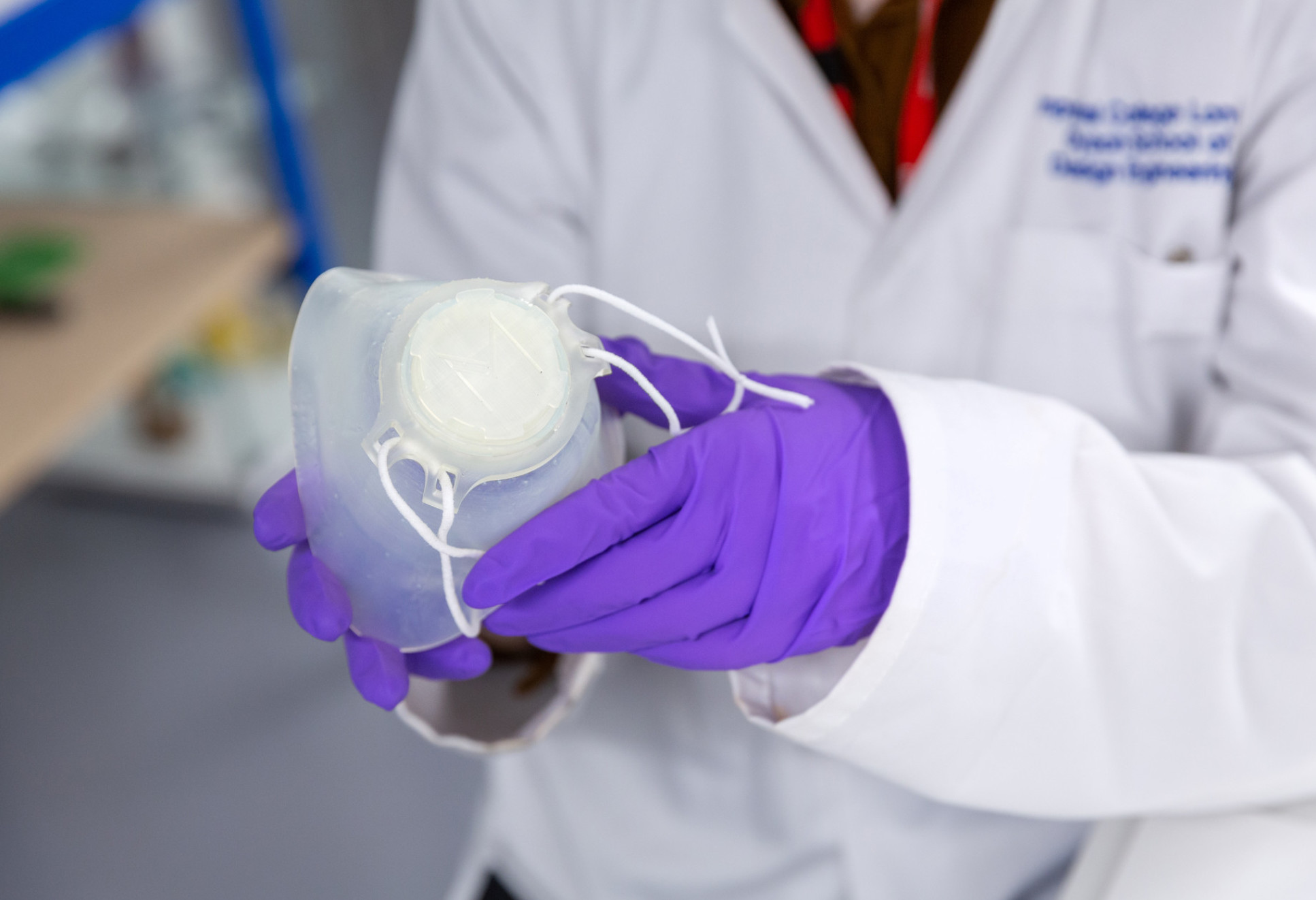
Facial characteristics vary in people of different ages, genders and ethnicities. In a workforce as diverse as the NHS, this means that a one-size-fits-all approach is not very effective, and current customisation options are expensive and time consuming.
Dr Myant will partner with PolyMetrix to utilise their technology for custom processing 3D models. Together they have developed an automated pipeline for the mass customisation of RPE which employs a 3D facial scan of the user, submitted via a web portal, to create fully customised RPE design files ready for manufacture within minutes. To date, the team have relied on third party scanning software but this latest funding will support the development of a 3D scanning app for seamless integration into their design pipeline.
Rapid diagnostic screening
Dr Jesus Rodriguez Manzano, from the Department of Infectious Disease, is leading a team developing rapid diagnostic screening for COVID-19 and other respiratory pathogens.
The COVID-19 pandemic has led to an unprecedented need for rapid diagnostic screening. Such screening involves the extraction and detection of viral RNA, which usually requires specialist laboratory equipment and trained personnel. This makes screening a challenge in low and middle income countries where access to the required equipment can be severely limited.

Dr Manzano’s team has developed a method which enables ultra-fast and inexpensive sample preparation without the need for specialist skills or equipment (SmartLid), previously funded by the Imperial's 3M COVID-19 Research Awards. In parallel, they have developed a low-cost colour-change detector which is stable at room temperature and can indicate a test result in less than 20 minutes. When coupled together their sample-to-result approach for COVID-19 detection has been named ‘DragonFly’.
This new funding will allow the team to expand DragonFly to rapidly extract and detect RNA from five other respiratory pathogens as well as COVID-19 - including both Flu viruses (A and B), Respiratory Syncytial Virus (RSV), SARS, and Rhinovirus (RV). It will also develop a smartphone app for image processing based on machine learning to allow for a rapid and objective analysis of the results.
Understanding severe illness in children
Dr Vanessa Sancho-Shimizu, from the Department of Infectious Disease, is working to understand the genetic basis for severe disease associated with COVID-19 in children.
Children are very unlikely to suffer from life-threatening COVID-19. However, last year researchers in the UK and several European countries with high numbers of COVID-19 cases also recognised a new inflammatory syndrome in children that was similar to Kawasaki disease, a rare syndrome known to affect young children. The extremely rare condition, which the researchers named Paediatric Inflammatory Multisystem Syndrome Temporally associated with SARS-CoV-2 (PIMS-TS), is associated with elevated inflammatory markers leading to, in some cases, myocardial injury shock and coronary artery aneurysms. Children with severe aneurysms are at life-long risk of heart attack.
Why some children suffer from severe disease whilst most children have very mild illness remains unknown. Dr Sancho-Schimizu will look to identify genetic variants that underlie severe COVID-19 disease and PIMS-TS to shed light on why some children get ill and with the potential to inform effective treatment options.
Detecting variants
Professor Paul Freemont, from the Department of Infectious Disease, is leading a project developing a platform for rapid detection of COVID-19 variants of concern using CRISPR-Cas systems.
As the focus of the pandemic shifts from diagnosis and treatment towards vaccination, there is a need for surveillance of variants that could compromise vaccination strategies. Current approaches, such as next generation sequencing, are too slow to provide clinically actionable results.
CRISPR-Cas is a technology that is widely known for its ability to make precise edits to genetic code by targeting specific sequences of DNA or RNA. This project could see CRISPR-Cas systems used to quickly search for fingerprints of mutations linked to COVID-19 variants. The team will investigate whether CRISPR-Cas systems can be engineered to detect variants and provide clinically actionable results while fitting within the current diagnostic pathways of NHS laboratories.
The team will also develop a library of variant "virus-like particles” - molecules that closely resemble viruses but are non-infectious because they contain only select parts of the virus' genome. These can be used to accurately mimic the characteristics of infectious virus without any biosafety concerns and provide a method to validate assays before clinical material is available. This repository could be used by researchers and companies around the world to develop assays for the detection of variants and the repository will be expanded in the future to serve as a resource for the development of diagnostics for other infectious diseases.
Strengthening international collaboration

The Jameel Fund is also funding complementary research projects at King Abdulaziz University in Jeddah, with a view to strengthening research collaborations between Imperial and Saudi universities in this field, as well as partnership projects at both institutions.
Dr Nishel Shah, from Imperial’s Department of Metabolism, Digestion and Reproduction, is collaborating with Dr Ahdab Alsaieedi, an Assistant Professor of Immunology at the Faculty of Applied Medical Sciences in King Abdulaziz University. The project will investigate how pregnancy affects immunity to COVID-19.
Pregnant women with COVID-19 are far more likely to develop severe illness, requiring hospital-based care and oxygen treatment, than women of a similar age who are not pregnant. Recent data suggests that the overall immunogenicity of COVID-19 vaccines (how well they provoke an immune response) in pregnant women is comparable to those who are not, although some aspects of these responses are altered. What is not clear currently is whether pregnancy affects how long these immune responses last.
The project will explore whether pregnancy-related immune changes affect how immunity develops as well as its longevity in pregnant women after either COVID-19 infection or vaccination.
Article supporters
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Deborah Evanson
Communications Division
Joanna Wilson
Communications Division