Publications and Case Studies
Publications
Jessica Wade, Francesco Salerno, Rachel C. Kilbride, Dong Kuk Kim, Julia A. Schmidt, Joel A. Smith, Luc M. LeBlanc, Emma H. Wolpert, Adebayo A. Adeleke, Erin R. Johnson, Jenny Nelson, Tadashi Mori, Kim E. Jelfs, Sandrine Heutz, Matthew J. Fuchter, Controlling anisotropic properties by manipulating the orientation of chiral small molecules, Nat. Chem., 2022, Accepted Manuscript.
Linden Schrecker, Joachim Dickhaut, Christian Holtze, Philipp Staehle, Marcel Vranceanu, Klaus Helgardt and King Kuok (Mimi) Hii, Discovery of unexpectedly complex reaction pathways for the Knorr pyrazole synthesis via transient flow, React. Chem. Eng., 2022, Accepted Manuscript.
- DOI: 10.1039/D2RE00271J
Roderick T. Stark, Dominic Pye, Wenyi Chen, Oliver J. Newton, Benjamin J. Deadman, Philip Miller, Jenny-Lee Panayides, Darren Lyall Riley, Klaus Hellgardt and King Kuok (Mimi) Hii, Assessing a Sustainable Manufacturing Route to Lapatinib, React. Chem. Eng., 2022, 7, 2420-2426.
- DOI: 10.1039/D2RE00267A
Benjamin J. Deadman, Sarah Gian, Violet Eng Yee Lee, Luis A. Adrio, Klaus Hellgardt and King Kuok (Mimi) Hii, On-demand, in situ, generation of ammonium caroate (peroxymonosulfate) for the dihydroxylation of alkenes to vicinal diols, Green Chem., 2022, 24, 5570-5578.
- DOI: 10.1039/D2GC00671E
- Data Repository: 10.14469/hpc/10417
Melinda Fekete, Toma Glasnov, King Kuok (Mimi) Hii and Benjamin J. Deadman, Technology overview/overview of the devices, in Flow Chemistry – Fundamentals, ed Ferenc Darvas, György Dormán, Volker Hessel and Steven V. Ley, De Gruyter, Berlin, 2nd edn, 2021, vol. 1, ch. 3, pp. 87-144.
David E. Hill, Jin-Quan Yu, and Donna G. Blackmond, Insights into the Role of Transient Chiral Mediators and Pyridone Ligands in Asymmetric Pd-Catalyzed C–H Functionalization, J. Org. Chem., 2020, 85, 13674-13679.
Case studies
Using reaction monitoring by IR to identify the active oxidant in the dihydroxylation of alkenes by peroxydisulfate
As reported in Green Chem., 2022, 24, 5570-5578, https://doi.org/10.1039/D2GC00671E
Whilst investigating the dihdroxylation of alkenes to vicinal diols by electrochemically generated ammonium peroxydisulfate (PDS), Dr Ben Deadman, Prof. Klaus Hellgardt and Prof. Mimi Hii observed that the reactivity of the oxidant increased after it had been aged overnight (Figure 1). This change in reactivity led the team to hypothesise that the active oxidant was actually the peroxymonosulfate (PMS) which would be generated in situ by the decomposition of PDS under acidic conditions.
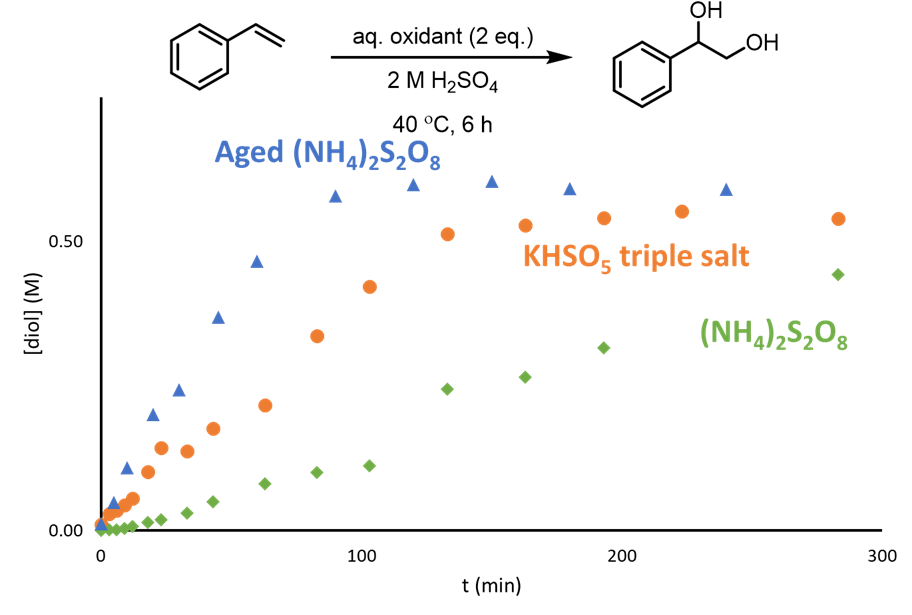
To confirm their hypothesis the team utilised a combination of the Mettler-Toledo ReactIR 15 and their bespoke redox colorimetric analysis method (React. Chem. Eng., 2017,2, 462-466) to follow the conversion of PDS to PMS under acidic aqueous conditions. The formation of PMS could be followed directly by the diagnostic IR absorbance at 760 cm-1 while the loss of PDS could also be followed by the IR absorbance at 1260cm-1 (Figure 2).
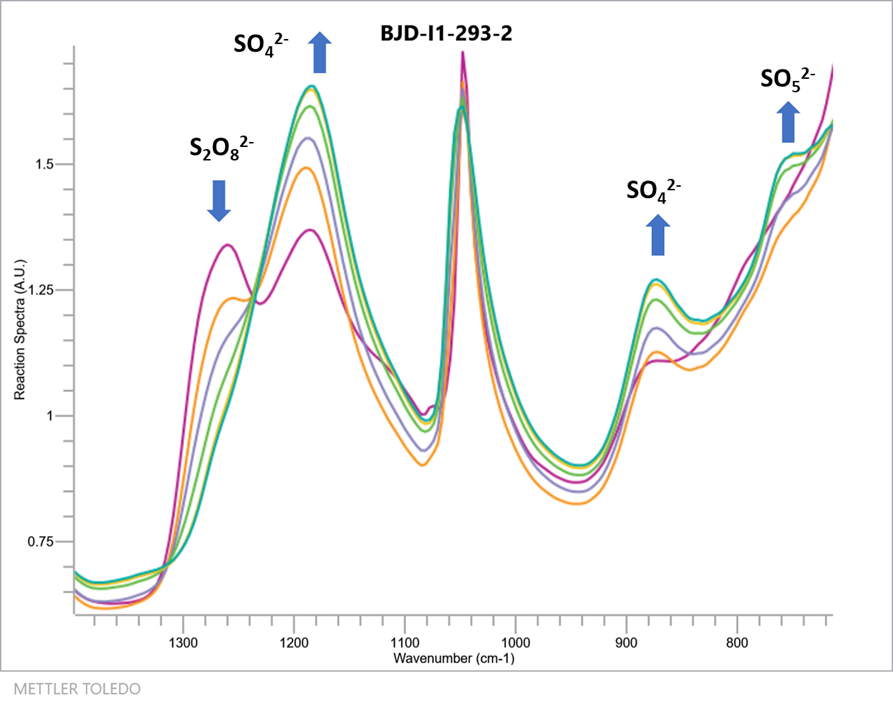
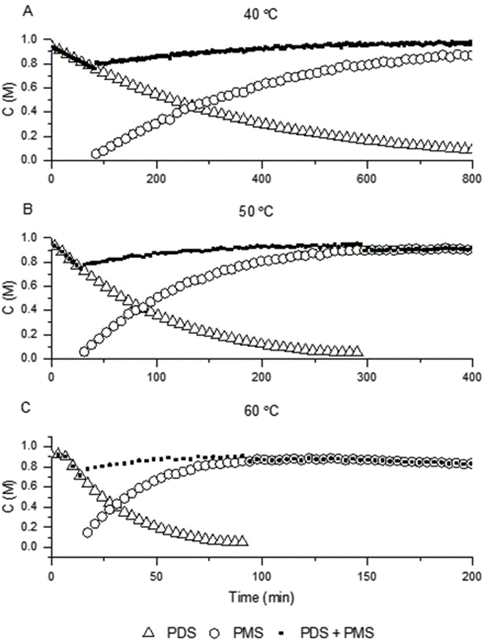
The team used the orthogonal IR and redox colorimetric methods to monitor the decomposition of PDS into PMS in acidic solutions at 40, 50 and 60 °C (Figure 3). Once formed, the PMS was observed to be remarkably stable at up to 50 °C, but increasing the temperature further led to the competitive decomposition of PMS to H2O2. Monitoring the reaction by IR also revealed the presence of a further intermediate through the delayed onset of PMS formation (tracked by plotting the sum of [PDS] and [PMS]). The time course data was then employed by the team to build a pseudo first-order kinetic model of the reaction, and obtain rate constants and an activation energy for the reaction process.
ROAR Capabilities and Services in this Case Study
- Mettler-Toledo ReactIR with 9.5 mm AgX DiComp™ batch probe
- Mettler-Toledo EaxyMax 102 reactor
- Custom reaction analysis method development
- Kinetic modelling of reaction processes


