Contact us
Level 2
Dr Victor Phillip Dahdaleh (VPD) Building
Hammersmith Hospital Campus
The Research Histology Facility is a key component of the Inflammation, Repair and Development Section at the NHLI, which extends its services to various Sections and Departments within Imperial and to external partners.
Our histology facility provides essential services, including the processing and embedding of tissue into paraffin wax blocks, sectioning and specialised staining. We also offer acryo-sectioning service and are able to provide guidance and training in histology techniques. We can also provide advice on protocol development and troubleshooting for immunohistochemistry staining. However, it is important to note that we do not offer an immunostaining service.
The IRD Histology Facility is run by Connor Preston, who has over twelve years experience working as a histologist in both NHS and research Laboratories.
Price List
|
Service
|
Unit
|
Tariff 1 NHLI Users |
Tariff 2 ICL Users |
Tariff 3 External Users |
|---|---|---|---|---|
| Process to Wax | Per cassette | £8.50 | £8.50 | £8.50 |
| Section Only | Per slide |
£4.00 |
£4.50 | £5.00 |
| Embed to Wax, Section & Stain | Per cassette |
£14.50 |
£15.50 | £16.50 |
| Stain Only (e.g. H&E) | Per slide |
£5.50 |
£6.00 |
£6.50 |
| Stain Only (Special) | Per slide | £10.00 | £10.50 | £11.50 |
| Cryosection | Per block | £25.00 | £27 | £30 |
| Hire of Microtome/Cryostat/Embedding Centre | Per hour |
£6 |
£8 | N/A – cost on application |
For full charging rates, please contact Connor Preston.
Services
Tissue processing prepares samples for microscopic study by removing water and replacing it with wax. A small piece of tissue is placed into a cassette and loaded into a tissue processor, which passes it through solutions (fixative, alcohol, clearing agent, and molten wax). This creates a firm, wax-impregnated sample suitable for embedding and cutting.
Embedding involves placing the processed tissue into a block of molten wax. Careful orientation is important to ensure that the correct tissue surface is exposed for sectioning. Once cooled, the wax block provides stable support, making it easier to cut extremely thin sections on a microtome.
Sectioning is the process of cutting thin slices of the embedded tissue using a microtome. Sections are usually cut at 3–5 microns in thickness and carefully transferred onto glass slides. This allows cellular structures to be clearly visualized under the microscope.
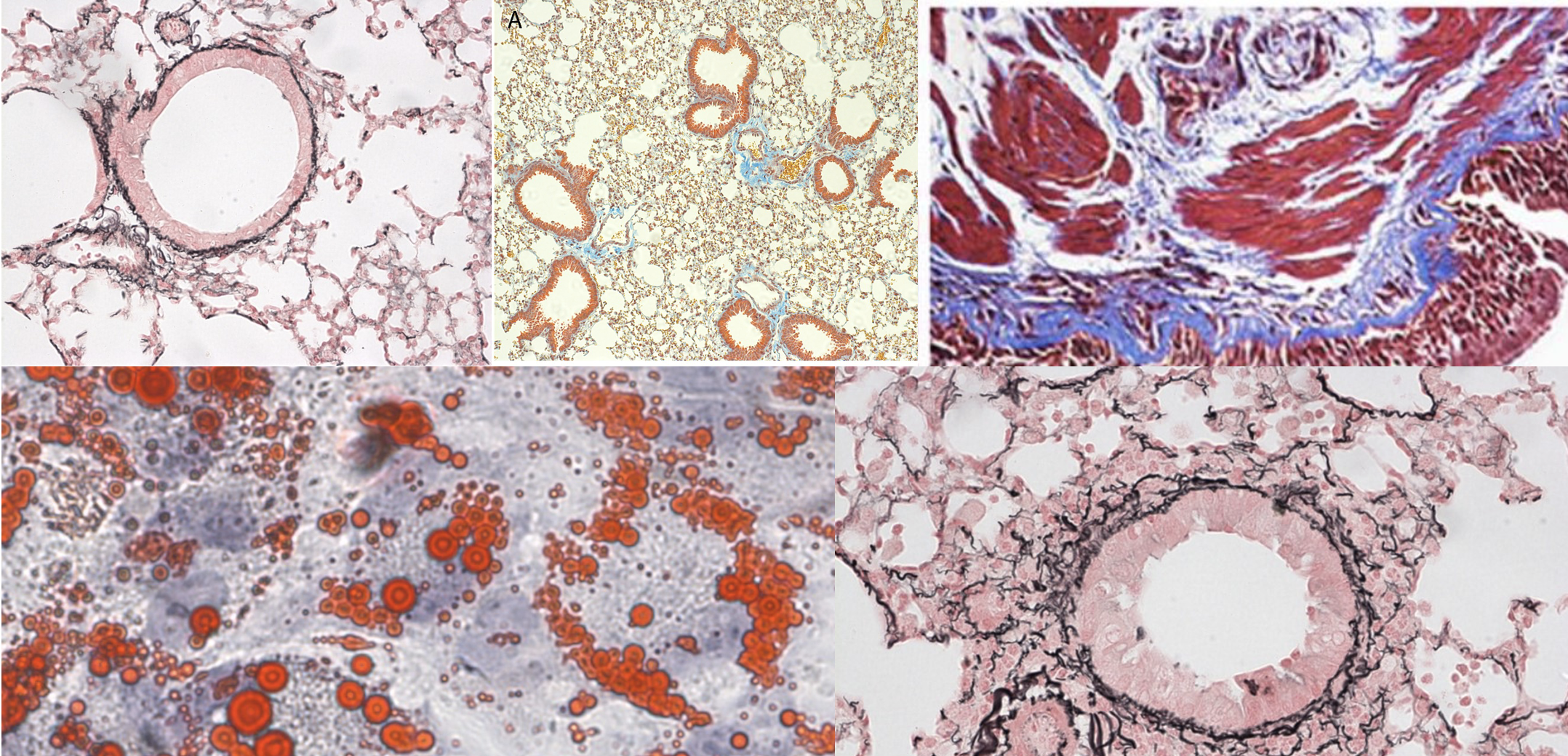
Staining is the final step that allows tissues to be seen clearly under the microscope.
The routine stain is:
- Haematoxylin & Eosin (H&E) → Haematoxylin stains nuclei blue-purple, showing cellular detail, while Eosin stains the cytoplasm and other structures pink. Together, H&E provides an excellent overview of tissue architecture and is the standard starting point for histological assessment.
In addition to H&E, we also offer a wide range of special stains to highlight particular tissue features or components. Examples include:
- EVG (Elastic Van Gieson) – demonstrates elastic fibres.
- Masson’s Trichrome – highlights connective tissue and collagen.
- Sirius Red – stains collagen fibres.
- PAS (Periodic Acid–Schiff) – shows carbohydrates, mucins, and basement membranes.
Other special stains are available on request, depending on diagnostic or research needs.
We now offer a range of histology equipment for hire, including:
- Microtomes – used to cut very thin sections of paraffin-embedded tissue for slide preparation.
- Cryostat – a specialized microtome housed in a refrigerated unit, used for cutting frozen tissue sections.
- Embedding Centre – used to embed processed tissue samples in wax blocks with correct orientation.
These instruments are essential for producing high-quality tissue sections for histological analysis.
Please note: proven competency or prior training is required to access both the facility and the equipment.
For more information or to discuss hire options, please contact Connor Preston.
Transport of specimens for wax processing
1. Fixatives are carcinogenic and toxic so specimens must never be transported in fixative owing to the danger of exposure in event of a leak.
2. Fix specimens according to protocol and using a fume hood transfer to 70% ethanol or IMS in a plastic screw top container. Note glass must not be used owing to danger of breakage.
3. Wrap the join between the lid and body of the jar with several turns of cling film.
4. Label the jar with contents and a contact name and number.
5. Insert the jar inside a double layer of resealable plastic bags (or a second screw top jar) to prevent leaks.
6. Transfer to the Histology lab at Hammersmith Hospital Campus inside another plastic bag.
Please note: It is important histology staff are contacted prior to delivering human specimens.
(Delivered to histology staff directly HTA requirements)