VEIN

VEIN Platform Trial Co-ordinating Centre
Section of Vascular Surgery
Room 14, 4th Floor East Wing
Charing Cross Hospital
Fulham Palace Road
London W6 8RF
Email: veinplatform@imperial.ac.uk
Venous leg ulcer (VLU) is the most severe manifestation of venous disease, with a prevalence of up to 4% in the elderly. Those affected have high rates of social isolation and depression with quality of life similar to end stage heart failure and increased mortality rates. VLU treatments present a tremendous burden on the healthcare service, with an estimated expenditure between £940 million and £1.3 billion per year.
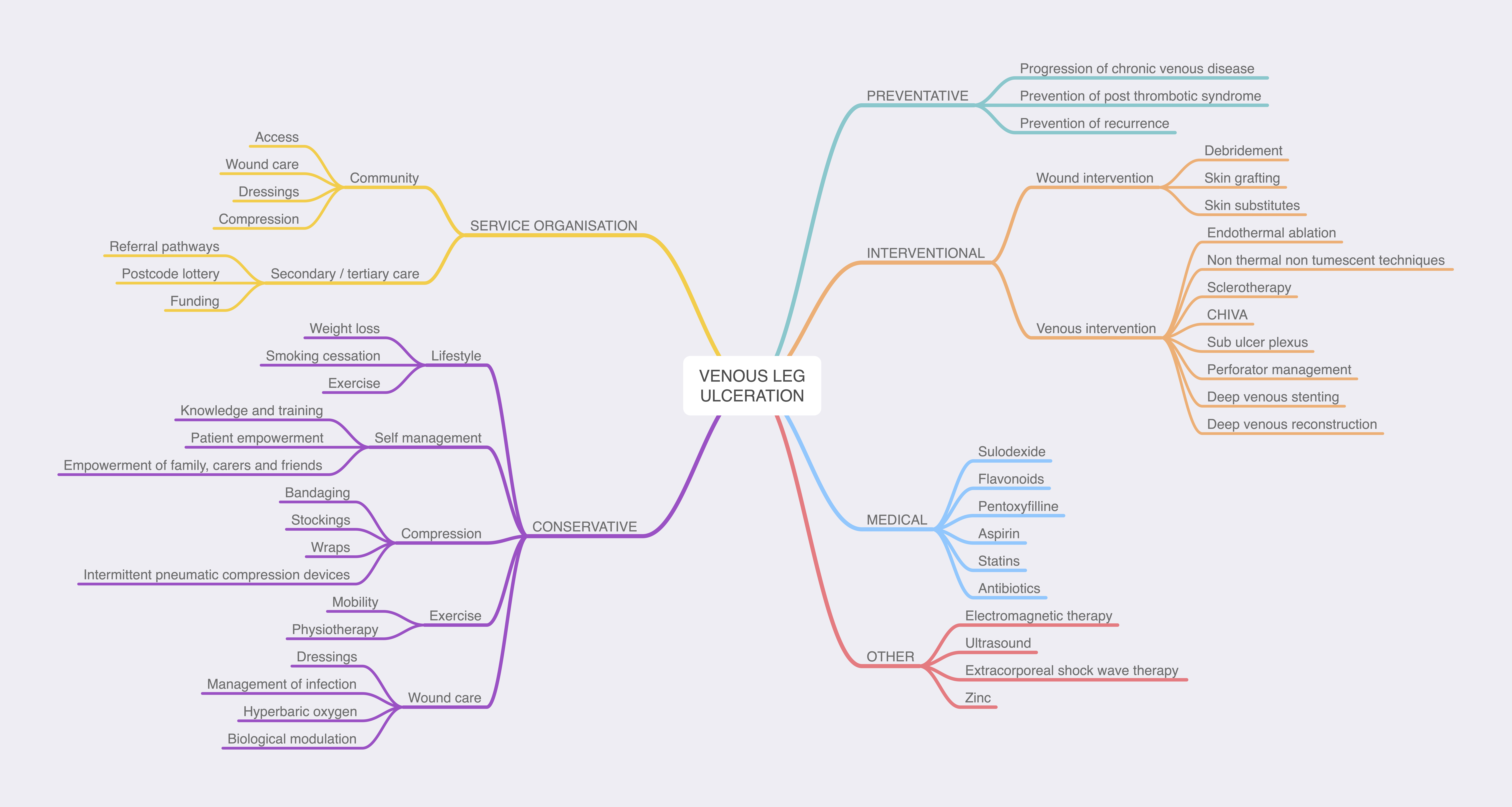
The pathophysiology of VLU is complex. Abnormalities of the superficial and/or deep venous systems can be responsible for VLU development. Management ranges from lifestyle measures to deep venous interventions; however, systematic reviews and guidelines report that evidence is of low quality. Where high quality evidence exists for specific interventions, healing rates and recurrence-prevention rates are still challenging to achieve. Numerous areas require urgent evaluation in a patient and disease-focussed process, which can potentially be delivered via a platform approach.
NIHR HTA funding application (2024)
In March 2024, a funding application was submitted to NIHR HTA for the VEIN Platform study which aims to deliver a multicentre platform trial assessing the benefit of multiple treatments compared to standard of care (SoC) across 3 clinical domains.
The accelerator grant award (please see further below) was used to inform the VEIN design:
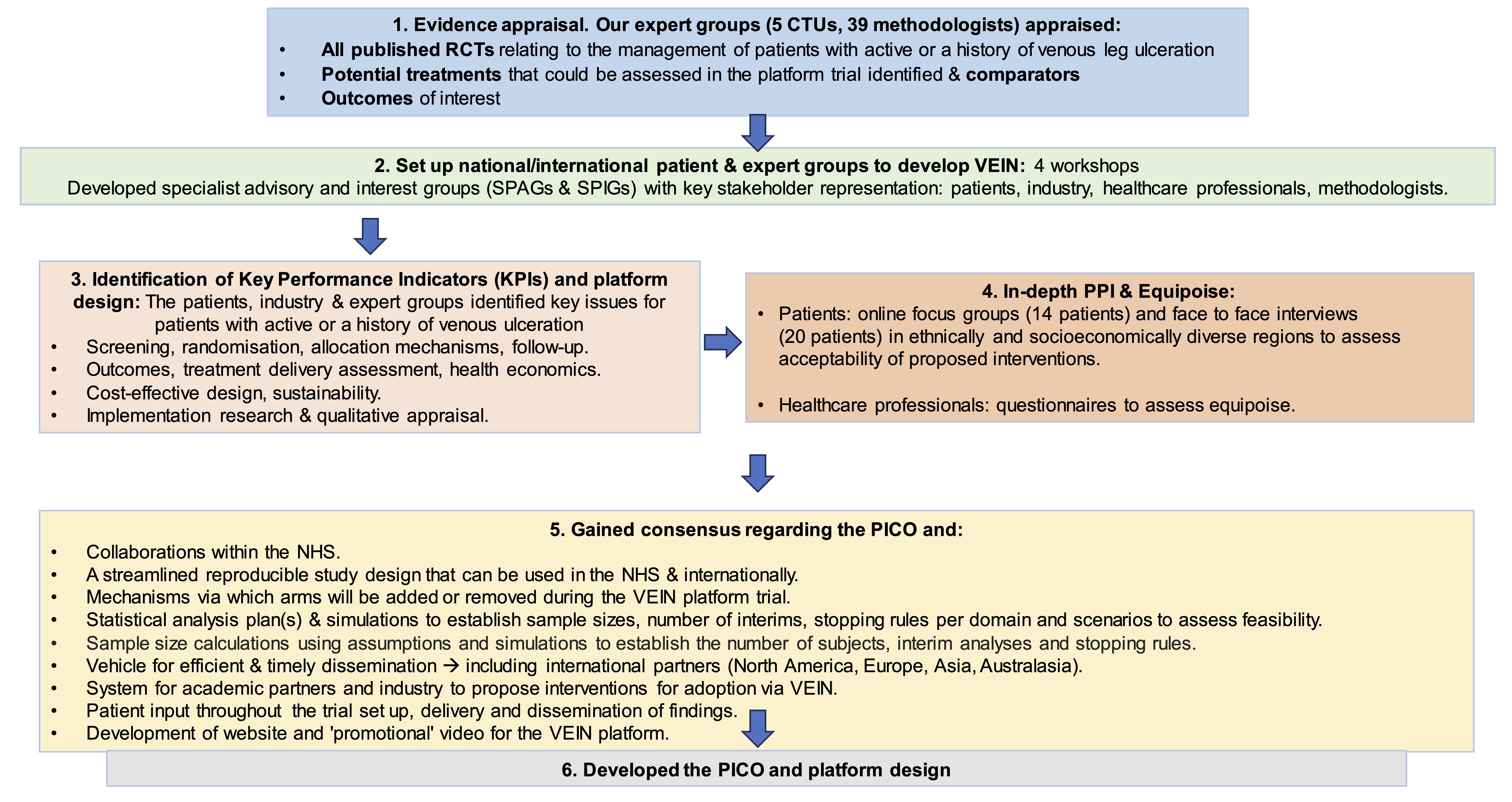
VEIN is a prospective randomised, controlled, multi-centre platform trial with 3 multi-arm-multistage (MAMS) domains determined by the clinical stage of disease. Three, four arm trials, randomised (1:1:1:1) will be delivered, incorporating new arms upon termination or completion of any intervention. Patients will be followed up as part of the study to determine whether open VLUs heal within six months, or whether healed VLUs recur.
About VEIN
Sample size
The initial platform design has maximum sample sizes of: 2,244 Domain 1, 1,320 Domain 2 and 3,395 Domain 3. For further information on sample size and optimisation, please click here.
Accelerator Grant Award (2022-2023)
Funder
National Institute for Health Research (NIHR) – Health Technology Assessment (HTA).
Research question
What is the optimal design and delivery strategy for a platform study evaluating the clinical and cost effectiveness of interventions for venous leg ulcer (VLU) prevention and healing?
Aims and objectives
To develop the research priorities, optimal design and infrastructure to allow the delivery of a future patient-focussed, platform trial assessing the clinical and cost-effectiveness of interventions for VLU treatment and prevention.
Methods
The study was delivered via five work streams (WS), which will: assess the evidence for VLU interventions, outcomes and comparators (WS1); establish a platform development group (PDG) with representation from patients, carers and their families, expert clinicians, industry partners, statisticians, health economists, data analytics and qualitative experts (WS2); identify key performance indicators, trial characteristics and infrastructure required to deliver the platform trial (WS3); finalise trial characteristics and implementation of platform infrastructure (WS4); finalise the funding application and trial protocol for submission (WS5).
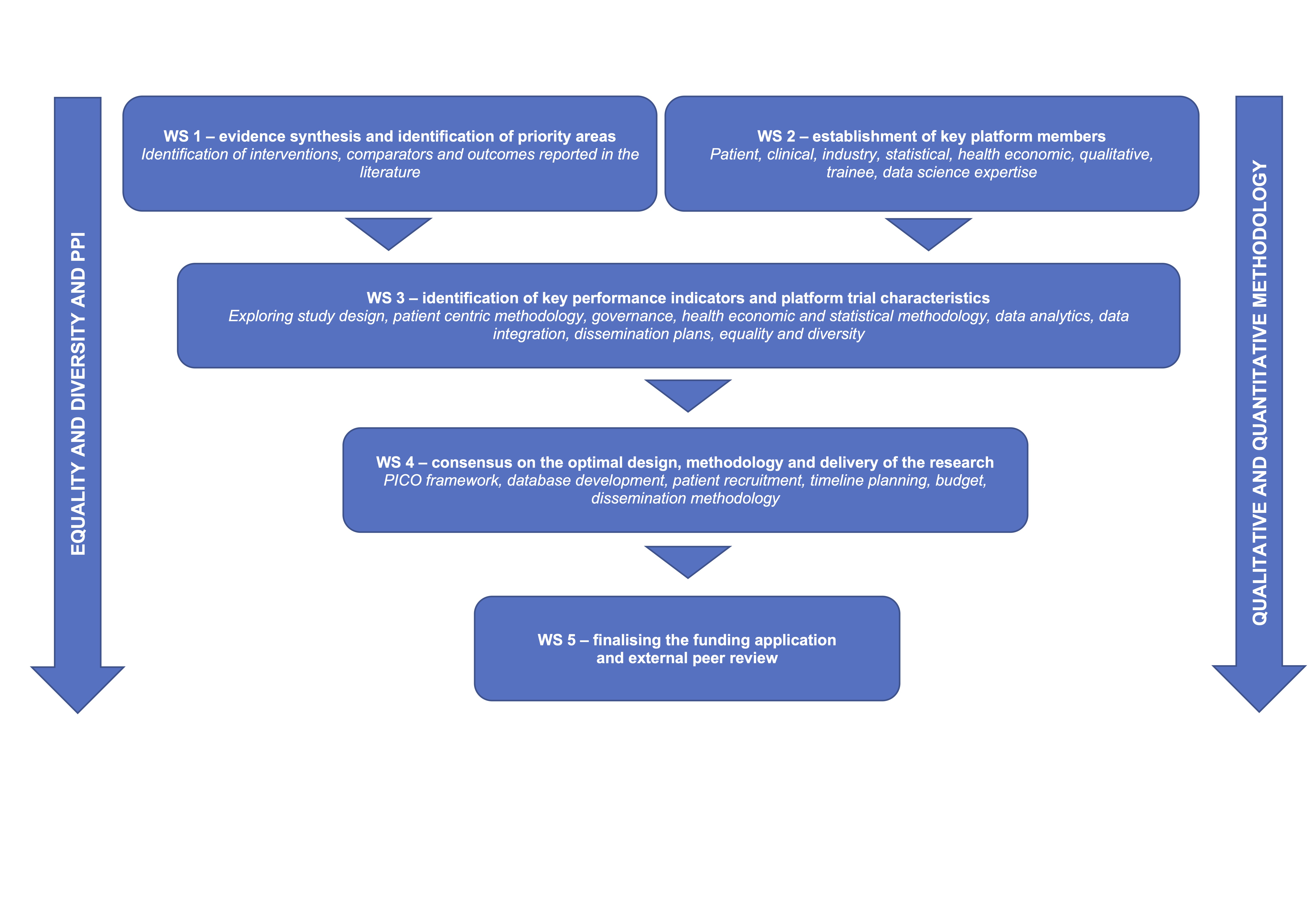
The streams were delivered via a mixed-method approach, including evidence synthesis, interviews, questionnaires, focus groups, Delphi consensus, statistical simulation, and cost-effectiveness model scoping and simulation.
The WS was delivered within 9 months to inform the NIHR HTA application in November 2023 (stage 1) and March 2024 (stage 2).
Platform development group
Core patient group (CPG): This is a group of patients, carers and family members (n ~ 10) who are the voice of VLU patients and continue to have active input throughout the study period, including in aspects such as: research question priority based on WS1, who they feel should be involved in the platform infrastructure (e.g. patients, allied health professionals); acceptability of study design and treatment; generation and review of patient facing information and research outputs. It is important that the CPG is representative and inclusive. We continue to adhere to NIHR-INVOLVE, INCLUDE and REPAG principles to maximise minority group recruitment and use different approaches, including advertising in the national press and social media (Facebook, Twitter, Instagram). Should we not achieve minority group representation, we will ensure lay members are recruited to help identify cultural and/or religious barriers to research participation.
Core clinical group: Clinicians with expertise in VLU management have been recruited to this group (n ~ 10). We performed a consultation exercise in preparation for this application to help inform the composition of this group. This will mirror the feedback received, with ~10 representatives from nursing, vascular surgery, dermatology, interventional radiology, appliance, physiotherapy, pharmacy and general practice. This group will provide clinical and practical expertise to support platform development, provide peer review on possible trial arms to be tested in the future study and comment on the acceptability of new technology (equipoise). They will provide input on clinician dissemination materials to avoid readership burden.
Core stakeholders: Representatives from industry, statisticians, health economists and other additional stakeholders committed to platform development who will meet regularly to advance platform planning. The Core group will undergo platform trial methodology and equality and diversity training.
Specialist Advisory Groups (SPAGs): Additional stakeholders who advise the core groups on platform development via surveys, questionnaires and focussed interviews, where appropriate.
Patient representatives: In addition to the CPG, who will be heavily involved in platform development, individuals interested in contributing to the platform are involved by completing questionnaires, undertaking focussed interviews, where appropriate, and providing anonymised feedback via a website interface, where preferable. This group will include patients, carers and their family members wishing to provide input into VLU research without committing to be part of the CPG, increasing the depth and breadth of patient feedback on the developing platform study.
Clinical representatives: This group includes representation of national and international societies championing VLU care and research, such as the European College of Phlebology, International Union of Phlebology, American Venous Forum, American Vein and Lymphatic Society, Vascular Society Special Interest Groups, National Wound Care Strategy Programme and Legs Matter. Representatives of the aforementioned bodies have already been contacted and are supportive of this research. Industry: Industry partners will be invited to participate in the PDG to support the introduction of medical devices and / or pharmacological agents in the trial arms as per pre-application questionnaire feedback.
Statistical expertise: We recognise the challenges in setting up and delivering platform trials. Hence, three established clinical trial units (CTU) will be collaborating on the project, including Imperial, Edinburgh and Leicester CTUs.
Health economics: This will be required to determine the cost effectiveness of different potential interventions. Experts from the University of Granada and the University of Edinburgh who have collaborated on large RCTs delivered by our group, will be part of the PMG.
Qualitative expertise: Qualitative methodology featured heavily during platform development. WS 3-6 relies on focus groups and structured interviews to help establish trial characteristics. Qualitative experts and behavioural health psychologists join appropriate meetings to identify themes and categories to ensure the maximum amount of data can be distilled from group encounters.
Trainee collaboratives: To promote trainee involvement in VLU research and aid delivery of the platform trial, trainee collaboratives were invited to participate, such as the Vascular and Endovascular Research Network (VERN). The Joint Lead Applicant is a VERN member (Onida). Trainee collaboratives help comment on feasibility and provide avenues to boost recruitment.
Data science: Evidence based health informatics (EBHI) is increasingly important in evidence appraisal and clinical trial methodology and delivery. It is expected that the platform study will generate large volumes of complex data, likely from different centres and countries. In addition, evidence appraisal will need to be integrated to ensure novel treatment modalities can be identified from the literature and incorporated in an efficient manner. Data analytical expertise will be sourced from the BHF Consortium and the Big Data Analytical Unit (BDAU) at Imperial College. These will help advise the optimal approach for data collection, management and analysis in a centralised, efficient and cost-effective fashion.
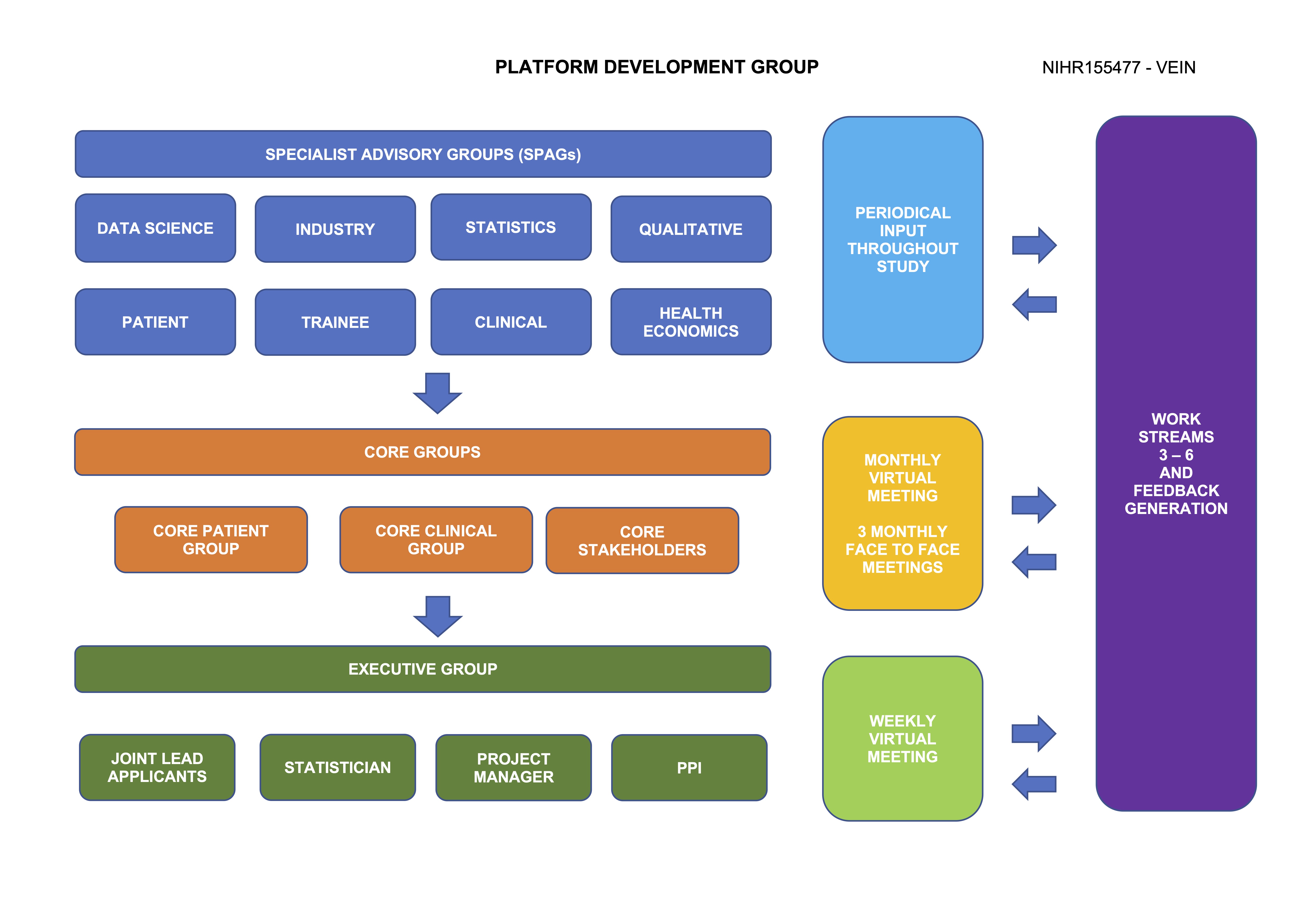
Specialist Interest Groups (SPIGs): Additional stakeholders who advise the core groups on the following areas of VLU management:
This project has developed the first VLU platform infrastructure, which will be used in the long term for VLU trials. Information regarding the platform will be relevant to and will inform patients, the public and clinicians. Regular updates drafted by lay members of the PDG will be delivered via the Vascular Research UK website, YouTube Channel and social media to the aforementioned groups, ensuring the information is understandable. Updates will also be provided to participating national and international bodies. The results will be presented at national and international conferences and published in peer reviewed journals. Regular updates during the study will be provided to core members of the team, with focus on patients and carers.
Trial co-ordinating centre staff
Professor Alun Davies
/prod01/channel_2/media/images/people-list-300X400/AHD2.jpg)
Professor Alun Davies
Chief Investigator
Miss Sarah Onida
/prod01/channel_2/media/images/people-list-300X400/Sarah_Onida--tojpeg_1544264129834_x1.jpeg)
Miss Sarah Onida
Chief Investigator
Dr Francine Heatley
/prod01/channel_2/media/images/people-list-300X400/Francine-Heatley-copy.png)
Dr Francine Heatley
Clinical Trials Manager
Team Profiles
- Professor Alun Davies, Professor of Vascular Surgery, Head of the Section of Vascular Surgery, Imperial College London
- Miss Sarah Onida, Honorary Senior Lecturer in Vascular Surgery, Imperial College London
- Dr Francine Heatley, Clinical Trial Project Manager, Imperial College London
- Mrs Natalia Klimowska-Nassar, Operations Manager - Clinical Research, Imperial College London
- Dr Rachel Phillips, Senior Lecturer in Medical Statistics and Clinical Trials, Imperial College London
- Mr Nicholas Johnson, Statistician / Research Fellow, Imperial College London
- Dr Ed Waddingham, Senior Statistician / Research Fellow, Imperial College London
- Professor John Norrie, Director ECTU, Professor of Medical Statistics, The University of Edinburgh
- Mr Daniel Carradice, Senior Lecturer in Vascular Surgery, University of Hull
- Professor Ian Chetter, Chair of Surgery, Hull University Teaching Hospitals NHS Trust
- Professor Athanasios Saratzis, Professor of Vascular Surgery, University of Leicester
- Dr Shaun Barber, Senior Statistician, University of Leicester
- Dr Babak Choodari-Oskooei, Principal Research Fellow and senior Statistician, University College London
- Professor Mahesh Parmar, Director of both the MRC Clinical Trials Unit, University College London
- Professor Colin Greaves, Professor of Psychology Applied to Health, University of Birmingham
- Professor Justin Waring, Professor of Medical Sociology & Healthcare Organisation, University of Birmingham
- Dr Thomas Withers, Research Fellow in Behaviour change, University of Birmingham
- Dr Barnaby Zoob Carter, Teaching Fellow - Module Lead for Sport, Exercise and Health Psychology, University of Birmingham
- Dr Gordon Moran, Simple Trials for Academic Research Lead, The University of Nottingham
- Dr Colin Crooks, Simple Trials for Academic Research Lead, The University of Nottingham
- Dr Marcus Jepson, Senior Lecturer in Qualitative Health Sciences, University of Bristol
- Dr David Epstein, Health Economist, University of Granada
- Mr Andrew Stoddart, Senior Health Economist, The University of Edinburgh
- Mr Andy Steptowe, PPI Representative
- Mrs Vivien Gibson, PPI Representative
- Dr David Wingfield, General Practitioner and Principal Research Fellow, Imperial College London
- Dr Adarsh Babber, GP Partner, Sheet Street Surgery
- Mr Adam Gwozdz, Clinical Lecturer in Vascular Surgery, Imperial College London
- Miss Layla Bolton-Saghdaoui, Vascular Clinical Nurse Specialist / Honorary Clinical Research Fellow, Imperial College London
- Professor Azeem Majeed, Professor of Primary Care and Public Health, Imperial College London
- Professor Stephen Black, Consultant Vascular Surgeon and Professor of Venous Surgery, Guys and St Thomas' NHS Trust
- Dr Narayan Karunanithy, Consultant Interventional Radiologist and Honorary Senior Lecturer, Guy's and St Thomas' NHS Foundation Trust
- Dr Leanne Atkin, Vascular Nurse Consultant, Mid Yorkshire Hospitals NHS Trust
- Professor Jo Dumville, Professor of Applied Health Research, The University of Manchester
- Dr Steven Rogers, NIHR Clinical Lecturer, The University of Manchester
- Professor Kavita Vedhara, Professor of Health Psychology, Cardiff University
- Dr Salvatore Di Puma, Lead Production Pharmacist, North Middlesex University Hospital NHS Trust
- Dr Bernard Ho, Consultant in Dermatology and Lymphoedema, St George's University Hospitals NHS Foundation Trust
- Dr Nina Al-saadi, Vascular Surgery Trainee, The Dudley Group NHS Foundation Trust
- Mr Manjit Gohel, Consultant Vascular & Endovascular Surgeon, Cambridge University Hospitals NHS Foundation Trust
- Professor Matthew Sydes, Associate Director of Data-Enabled Trials (Prof of Clinical Trials and Methodology), Health Data Research UK
Find further details on the VEIN co-applicants, including biographies, here.


