Video for the catalysis activity
The Science
Catalysis is a process that chemists use to increase the rate of chemical reactions, using a unique substance called a catalyst. Sometimes, the use of catalysts can be essential for certain chemical processes because it allows a reaction to happen at moderate conditions without excessive use of energy. For example, in order to turn cellulosic biomass (think about really fibrous wood pulp) into biofuels, catalysts are used to break it down to smaller molecules. Otherwise, scientists would need to put it in reactors like pressure cookers at very high temperature! That is not ideal in a world where we try to save energy.
In our daily lives, catalysts are also commonly used in the kitchen for fermentation. This is a natural process where enzymes, or biological catalysts, breakdown complex compounds into simpler ones that are also easier to digest. The enzyme that turns milk into yoghurt is a great example. This is a reaction where lactose (a type of sugar in milk) is converted to lactic acid, where the sour flavour comes from. Breadmaking involves another reaction where glucose (a type of sugar in flour) is converted to ethanol (boiled-off in baking) and carbon dioxide, the gas that creates the tiny holes in bread. The following is the basic description of the reaction:
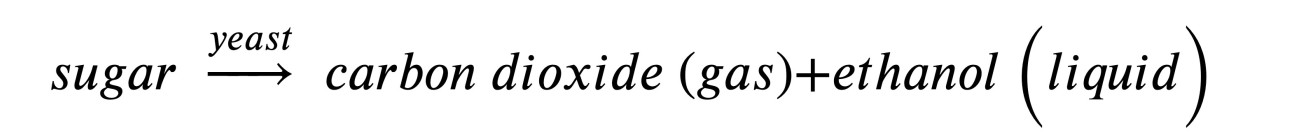
In this experiment, we will look at how the baker’s yeast works as a catalyst. Make sure to read through the entire process before you start.
Materials
- Water
- 2 sachets of fast action dry yeast (each 7g)
- Sugar
- 3 bottles (500ml)
- 3 balloons (ideally size 7inches or 175mm)
- Tablespoon
- Kettle
- Funnel (make sure it’s dry)
- Marker
- Stopwatch or time
Method
- Label the bottles as A, B, C with the marker.
- Use the funnel to add 2 tablespoons of sugar in each of the three bottles. If you do not own a funnel, you can create a cone with a piece of paper.
- Add one sachet of yeast each in bottles B and C, leaving bottle A without.
- Prepare some warm water by combining one part boiling water and two parts cold water. If it is hotter than roughly 38°C, we risk killing the yeast. To be certain, you can use a thermometer if you have one.
Follow steps 5 to 7 below for each bottle. You want to get the balloon on as quickly as possible after you swirl. For bottles A and B use room temperature water, for bottle C use the warm water you prepared.
- Add one cup (roughly 230ml) water.
- Carefully swirl the bottles to mix.
- Put a balloon on the neck of the bottle and start a stopwatch (or note time already elapsed).
- Let the bottles sit for 20 minutes and observe throughout. You may wish to give them another swirl every five minutes.
Things to think about
While you observe the reactions, try to answer the following questions:
- How would you determine whether the reaction is happening?
- What do you observe in the bottles and balloons after 5 minutes, 10 minutes, 15 minutes, 20 minutes, and beyond? Scientists studying reactions would often need to make observations at different time points to compare the rate of reaction.
- Comparing bottles A and B, what is the effect of the yeast?
- Comparing bottles B and C, what is the effect of the warm water?
- Does the balloon stop growing bigger eventually? Why?
- How would you tweak the recipe to speed up the reaction, by changing for example, the amount of yeast, sugar, or water temperature?
- How would you tweak the recipe to make the balloons bigger over time?
Notes
- Bottle A is considered a scientific control, which is designed to minimise the effects of factors other than the ones that you are studying. The water added would be a common factor. We want to investigate whether the reaction happening in bottle B is indeed caused by the yeast, and not just the water itself.
- A reaction is a process where reactant(s) transform into product(s). The reaction is complete when all the reactants are consumed. In this case, the reactant is the sugar. The yeast, on the other hand, is a catalyst, which helps the reaction proceed, but is not consumed. To continue the reaction, you could try removing the balloon of one of the complete reactions, add more sugar to the bottle, and put the balloon back on.
Caution
When using kettle to make warm water use care and have adult supervision
Outreach Newsletter
Sign up to our newsletter and mailing list for updates about Outreach events and activities.